Altered Micafungin Pharmacokinetics in Intensive Care Unit Patients
- PMID: 25963988
- PMCID: PMC4505244
- DOI: 10.1128/AAC.00623-15
Altered Micafungin Pharmacokinetics in Intensive Care Unit Patients
Abstract
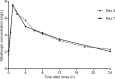
Micafungin is considered an important agent for the treatment of invasive fungal infections in the intensive care unit (ICU). Little is known on the pharmacokinetics of micafungin. We investigated micafungin pharmacokinetics (PK) in ICU patients and set out to explore the parameters that influence micafungin plasma concentrations. ICU patients receiving 100 mg of intravenous micafungin once daily for suspected or proven fungal infection or as prophylaxis were eligible. Daily trough concentrations and PK curves (days 3 and 7) were collected. Pharmacokinetic analysis was performed using a standard two-stage approach. Twenty patients from the ICUs of four hospitals were evaluated. On day 3 (n = 20), the median (interquartile range [IQR]) area under the concentration-time curve from 0 to 24 h (AUC0-24) was 78.6 (65.3 to 94.1) mg · h/liter, the maximum concentration of drug in serum (Cmax) was 7.2 (5.4 to 9.2) mg/liter, the concentration 24 h after dosing (C24) was 1.55 (1.4 to 3.1) mg/liter, the volume of distribution (V) was 25.6 (21.3 to 29.1) liters, the clearance (CL) was 1.3 (1.1 to 1.5) liters/h, and the elimination half-life (t1/2) was 13.7 (12.2 to 15.5) h. The pharmacokinetic parameters on day 7 (n = 12) were not significantly different from those on day 3. Daily trough concentrations (day 3 to the end of therapy) showed moderate interindividual (57.9%) and limited intraindividual variability (12.9%). No covariates of the influence on micafungin exposure were identified. Micafungin was considered safe and well tolerated. We performed the first PK study with very intensive sampling on multiple occasions in ICU patients, which aided in resolving micafungin PK. Strikingly, micafungin exposure in our cohort of ICU patients was lower than that in healthy volunteers but not significantly different from that of other reference populations. The clinical consequence of these findings must be investigated in a pharmacokinetic-pharmacodynamic (PK-PD) study incorporating outcome in a larger cohort. (This study is registered at ClinicalTrials.gov under registration no. NCT01783379.).
Copyright © 2015, American Society for Microbiology. All Rights Reserved.
Figures



References
-
- Vincent JL, Rello J, Marshall J, Silva E, Anzueto A, Martin CD, Moreno R, Lipman J, Gomersall C, Sakr Y, Reinhart K, EPIC II Group Investigators . 2009. International study of the prevalence and outcomes of infection in intensive care units. JAMA 302:2323–2329. doi:10.1001/jama.2009.1754. - DOI - PubMed
-
- Kett DH, Azoulay E, Echeverria PM, Vincent JL, Extended Prevalence of Infection in ICU Study (EPIC II) Group of Investigators. 2011. Candida bloodstream infections in intensive care units: analysis of the extended prevalence of infection in intensive care unit study. Crit Care Med 39:665–670. doi:10.1097/CCM.0b013e318206c1ca. - DOI - PubMed
-
- Cornely OA, Bassetti M, Calandra T, Garbino J, Kullberg BJ, Lortholary O, Meersseman W, Akova M, Arendrup MC, Arikan-Akdagli S, Bille J, Castagnola E, Cuenca-Estrella M, Donnelly JP, Groll AH, Herbrecht R, Hope WW, Jensen HE, Lass-Florl C, Petrikkos G, Richardson MD, Roilides E, Verweij PE, Viscoli C, Ullmann AJ, ESCMID Fungal Infection Study Group. 2012. ESCMID* guideline for the diagnosis and management of Candida diseases 2012: non-neutropenic adult patients. Clin Microbiol Infect 18(Suppl 7):S19–S37. doi:10.1111/1469-0691.12039. - DOI - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical

