Human endometrial mesenchymal stem cells restore ovarian function through improving the renewal of germline stem cells in a mouse model of premature ovarian failure
- PMID: 25964118
- PMCID: PMC4490699
- DOI: 10.1186/s12967-015-0516-y
Human endometrial mesenchymal stem cells restore ovarian function through improving the renewal of germline stem cells in a mouse model of premature ovarian failure
Abstract
Background: Human endometrial mesenchymal stem cells (EnSCs) derived from menstrual blood have mesenchymal stem/stromal cells (MSCs) characteristics and can differentiate into cell types that arise from all three germ layers. We hypothesized that EnSCs may offer promise for restoration of ovarian dysfunction associated with premature ovarian failure/insufficiency (POF/POI).
Methods: Mouse ovaries were injured with busulfan and cyclophosphamide (B/C) to create a damaged ovary mouse model. Transplanted EnSCs were injected into the tail vein of sterilized mice (Chemoablated with EnSCs group; n = 80), or culture medium was injected into the sterilized mice via the tail vein as chemoablated group (n = 80). Non-sterilized mice were untreated controls (n = 80). Overall ovarian function was measured using vaginal smears, live imaging, mating trials and immunohistochemical techniques.
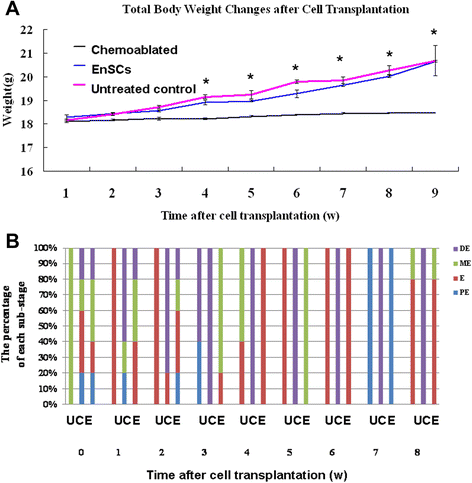
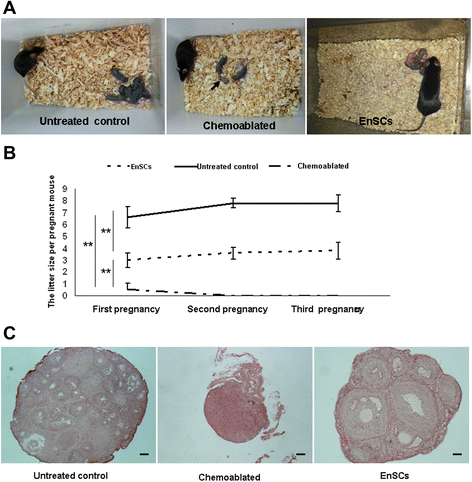
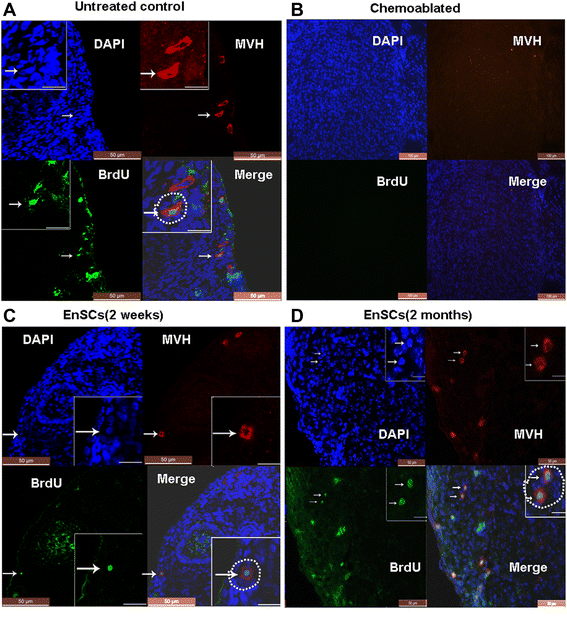
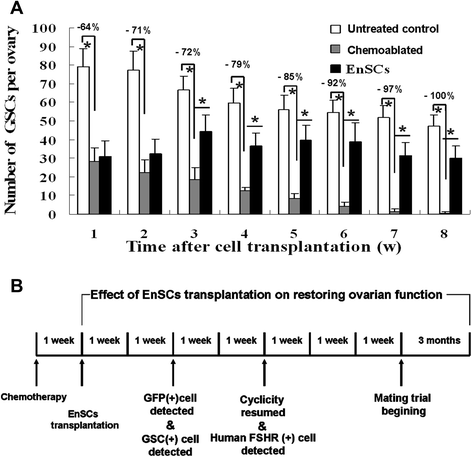
Results: EnSCs transplantation increased body weight and improved estrous cyclicity as well as restored fertility in sterilized mice. Migration and localization of GFP-labeled EnSCs as measured by live imaging and immunofluorescent methods indicated that GFP-labeled cells were undetectable 48 h after cell transplantation, but were later detected in and localized to the ovarian stroma. 5'-bromodeoxyuridine (BrdU) and mouse vasa homologue (MVH) protein double-positive cells were immunohistochemically detected in mouse ovaries, and EnSC transplantation reduced depletion of the germline stem cell (GSCs) pool induced by chemotherapy.
Conclusion: EnSCs derived from menstrual blood, as autologous stem cells, may restore damaged ovarian function and offer a suitable clinical strategy for regenerative medicine.
Figures



Similar articles
-
Transplantation of adipose-derived stem cells combined with collagen scaffolds restores ovarian function in a rat model of premature ovarian insufficiency.Hum Reprod. 2016 May;31(5):1075-86. doi: 10.1093/humrep/dew041. Epub 2016 Mar 9. Hum Reprod. 2016. PMID: 26965432
-
Tracking of human embryonic stem cell-derived mesenchymal stem cells in premature ovarian failure model mice.Biochem Biophys Res Commun. 2021 Nov 5;577:6-11. doi: 10.1016/j.bbrc.2021.08.063. Epub 2021 Aug 26. Biochem Biophys Res Commun. 2021. PMID: 34487961
-
Study of the reparative effects of menstrual-derived stem cells on premature ovarian failure in mice.Stem Cell Res Ther. 2017 Jan 23;8(1):11. doi: 10.1186/s13287-016-0458-1. Stem Cell Res Ther. 2017. PMID: 28114977 Free PMC article.
-
Premature ovarian insufficiency: pathogenesis and therapeutic potential of mesenchymal stem cell.J Mol Med (Berl). 2021 May;99(5):637-650. doi: 10.1007/s00109-021-02055-5. Epub 2021 Feb 27. J Mol Med (Berl). 2021. PMID: 33641066 Review.
-
Ovarian stem cell niche and follicular renewal in mammals.Anat Rec (Hoboken). 2011 Aug;294(8):1284-306. doi: 10.1002/ar.21422. Epub 2011 Jun 28. Anat Rec (Hoboken). 2011. PMID: 21714105 Review.
Cited by
-
Current Status and Future Prospects of Stem Cell Therapy for Infertile Patients with Premature Ovarian Insufficiency.Biomolecules. 2024 Feb 19;14(2):242. doi: 10.3390/biom14020242. Biomolecules. 2024. PMID: 38397479 Free PMC article. Review.
-
Non-gynaecological Applications of Menstrual-derived Stem Cells: A Systematic Review.Avicenna J Med Biotechnol. 2022 Jan-Mar;14(1):10-29. doi: 10.18502/ajmb.v14i1.8166. Avicenna J Med Biotechnol. 2022. PMID: 35509365 Free PMC article. Review.
-
Effects of low intensity pulsed ultrasound on expression of B-cell lymphoma-2 and BCL2-Associated X in premature ovarian failure mice induced by 4-vinylcyclohexene diepoxide.Reprod Biol Endocrinol. 2021 Jul 20;19(1):113. doi: 10.1186/s12958-021-00799-w. Reprod Biol Endocrinol. 2021. PMID: 34284777 Free PMC article.
-
VSELs may obviate cryobanking of gonadal tissue in cancer patients for fertility preservation.J Ovarian Res. 2015 Nov 17;8:75. doi: 10.1186/s13048-015-0199-2. J Ovarian Res. 2015. PMID: 26576728 Free PMC article.
-
Mesenchymal Stem Cells in Premature Ovarian Insufficiency: Mechanisms and Prospects.Front Cell Dev Biol. 2021 Aug 3;9:718192. doi: 10.3389/fcell.2021.718192. eCollection 2021. Front Cell Dev Biol. 2021. PMID: 34414193 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

