Tamoxifen regulation of sphingolipid metabolism--Therapeutic implications
- PMID: 25964209
- PMCID: PMC4516673
- DOI: 10.1016/j.bbalip.2015.05.001
Tamoxifen regulation of sphingolipid metabolism--Therapeutic implications
Abstract
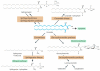
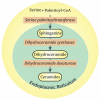
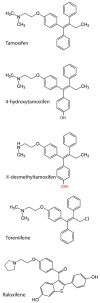
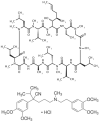
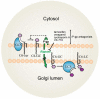
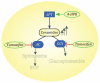
Tamoxifen, a triphenylethylene antiestrogen and one of the first-line endocrine therapies used to treat estrogen receptor-positive breast cancer, has a number of interesting, off-target effects, and among these is the inhibition of sphingolipid metabolism. More specifically, tamoxifen inhibits ceramide glycosylation, and enzymatic step that can adventitiously support the influential tumor-suppressor properties of ceramide, the aliphatic backbone of sphingolipids. Additionally, tamoxifen and metabolites N-desmethyltamoxifen and 4-hydroxytamoxifen, have been shown to inhibit ceramide hydrolysis by the enzyme acid ceramidase. This particular intervention slows ceramide destruction and thereby depresses formation of sphingosine 1-phosphate, a mitogenic sphingolipid with cancer growth-promoting properties. As ceramide-centric therapies are becoming appealing clinical interventions in the treatment of cancer, agents like tamoxifen that can retard the generation of mitogenic sphingolipids and buffer ceramide clearance via inhibition of glycosylation, take on new importance. In this review, we present an abridged, lay introduction to sphingolipid metabolism, briefly chronicle tamoxifen's history in the clinic, examine studies that demonstrate the impact of triphenylethylenes on sphingolipid metabolism in cancer cells, and canvass works relevant to the use of tamoxifen as adjuvant to drive ceramide-centric therapies in cancer treatment. The objective is to inform the readership of what could be a novel, off-label indication of tamoxifen and structurally-related triphenylethylenes, an indication divorced from estrogen receptor status and one with application in drug resistance.
Keywords: Acid ceramidase; Glucosylceramide synthase; Multidrug resistance; P-glycoprotein; Sphingolipid; Tamoxifen.
Copyright © 2015 Elsevier B.V. All rights reserved.
Figures
References
-
- Morad SA, Cabot MC. Ceramide-orchestrated signalling in cancer cells. Nat Rev Cancer. 2013;13:51–65. - PubMed
-
- Saddoughi SA, Ogretmen B. Diverse functions of ceramide in cancer cell death and proliferation. Adv Cancer Res. 2013;117:37–58. - PubMed
-
- Lavie Y, Cao H, Bursten SL, Giuliano AE, Cabot MC. Accumulation of glucosylceramides in multidrug-resistant cancer cells. J Biol Chem. 1996;271:19530–19536. - PubMed
-
- Morjani H, Aouali N, Belhoussine R, Veldman RJ, Levade T, Manfait M. Elevation of glucosylceramide in multidrug-resistant cancer cells and accumulation in cytoplasmic droplets. Int J Cancer. 2001;94:157–165. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

