Hepatitis B Virus Screening for Patients With Cancer Before Therapy: American Society of Clinical Oncology Provisional Clinical Opinion Update
- PMID: 25964247
- PMCID: PMC4477791
- DOI: 10.1200/JCO.2015.61.3745
Hepatitis B Virus Screening for Patients With Cancer Before Therapy: American Society of Clinical Oncology Provisional Clinical Opinion Update
Abstract
Purpose: This updated provisional clinical opinion presents a revised opinion based on American Society of Clinical Oncology panel consensus in the context of an evolving database.
Context: Despite the 2010 provisional clinical opinion recommendation, there is still evidence of suboptimal hepatitis B virus (HBV) screening among patients at high risk for HBV infection or HBV reactivation after chemotherapy. This updated provisional clinical opinion introduces a risk-adaptive strategy to identify and treat patients with HBV infection to reduce their risk of HBV reactivation.
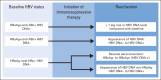
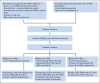
Provisional clinical opinion: Medical providers should screen by testing patients for HBV infection before starting anti-CD20 therapy or hematopoietic cell transplantation. Providers should also screen patients with risk factors for HBV infection. Screening should include both hepatitis B surface antigen (HBsAg) and hepatitis B core antibody (anti-HBc), because reactivation can occur in patients who are HBsAg positive/anti-HBc positive or HBsAg negative/anti-HBc positive. Either total anti-HBc or anti-HBc immunoglobulin G (not immunoglobulin M) test should be used. Clinicians should start antiviral therapy for HBsAg-positive/anti-HBc-positive patients before or contemporaneously with cancer therapy and monitor HBsAg-negative/anti-HBc-positive patients for reactivation with HBV DNA and ALT levels, promptly starting antivirals if reactivation occurs. Clinicians can initiate antivirals for HBsAg-negative/anti-HBc-positive patients anticipating cancer therapies associated with a high risk of reactivation, or they can monitor HBV DNA and ALT levels and initiate on-demand antivirals. For patients who neither have HBV risk factors nor anticipate cancer therapy associated with a high risk of reactivation, current evidence does not support HBV screening before initiation of cancer therapy. Two panel members provided a minority viewpoint, involving a strategy of universal HBsAg and selective anti-HBc testing.
© 2015 by American Society of Clinical Oncology.
Conflict of interest statement
Authors' disclosures of potential conflicts of interest are found in the article online at
Figures
Comment in
-
Computerized Physician Order Entry-Based System Improves Hepatitis B Virus Screening in Patients Undergoing Chemotherapy.J Clin Oncol. 2016 Jan 20;34(3):290. doi: 10.1200/JCO.2015.63.6779. Epub 2015 Dec 7. J Clin Oncol. 2016. PMID: 26644539 No abstract available.
-
Reply to J. Cabezas et al.J Clin Oncol. 2016 Jan 20;34(3):290-1. doi: 10.1200/JCO.2015.64.3064. Epub 2015 Dec 7. J Clin Oncol. 2016. PMID: 26644541 No abstract available.
References
-
- Artz AS, Somerfield MR, Feld JJ, et al. American Society of Clinical Oncology provisional clinical opinion: Chronic hepatitis B virus infection screening in patients receiving cytotoxic chemotherapy for treatment of malignant diseases. J Clin Oncol. 2010;28:3199–3202. - PubMed
-
- US Department of Health and Human Services. Action Plan for the Prevention, Care and Treatment of Viral Hepatitis: Updated, 2014-2016. http://aids.gov/pdf/viral-hepatitis-action-plan.pdf.
-
- US Food and Drug Administration. FDA drug safety communication: Boxed warning and new recommendations to decrease risk of hepatitis B reactivation with the immune-suppressing and anti-cancer drugs Arzerra (ofatumumab) and Rituxan (rituximab) http://www.fda.gov/Drugs/DrugSafety/ucm366406.htm.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

