Silver nanoparticles: correlating nanoparticle size and cellular uptake with genotoxicity
- PMID: 25964273
- PMCID: PMC4566096
- DOI: 10.1093/mutage/gev020
Silver nanoparticles: correlating nanoparticle size and cellular uptake with genotoxicity
Abstract
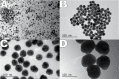
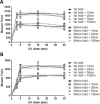
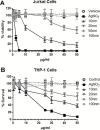
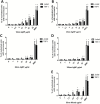
The focus of this research was to develop a better understanding of the pertinent physico-chemical properties of silver nanoparticles (AgNPs) that affect genotoxicity, specifically how cellular uptake influences a genotoxic cell response. The genotoxicity of AgNPs was assessed for three potential mechanisms: mutagenicity, clastogenicity and DNA strand-break-based DNA damage. Mutagenicity (reverse mutation assay) was assessed in five bacterial strains of Salmonella typhimurium and Echerichia coli, including TA102 that is sensitive to oxidative DNA damage. AgNPs of all sizes tested (10, 20, 50 and 100nm), along with silver nitrate (AgNO3), were negative for mutagenicity in bacteria. No AgNPs could be identified within the bacteria cells using transmission electron microscopy (TEM), indicating these bacteria lack the ability to actively uptake AgNPs 10nm or larger. Clastogenicity (flow cytometry-based micronucleus assay) and intermediate DNA damage (DNA strand breaks as measured in the Comet assay) were assessed in two mammalian white blood cell lines: Jurkat Clone E6-1 and THP-1. It was observed that micronucleus and Comet assay end points were inversely correlated with AgNP size, with smaller NPs inducing a more genotoxic response. TEM results indicated that AgNPs were confined within intracellular vesicles of mammalian cells and did not penetrate the nucleus. The genotoxicity test results and the effect of AgNO3 controls suggest that silver ions may be the primary, and perhaps only, cause of genotoxicity. Furthermore, since AgNO3 was not mutagenic in the gram-negative bacterial Ames strains tested, the lack of bacterial uptake of the AgNPs may not be the major reason for the lack of genotoxicity observed.
Published by Oxford University Press on behalf of the UK Environmental Mutagen Society 2015.
Figures

Similar articles
-
Size- and coating-dependent cytotoxicity and genotoxicity of silver nanoparticles evaluated using in vitro standard assays.Nanotoxicology. 2016 Nov;10(9):1373-84. doi: 10.1080/17435390.2016.1214764. Epub 2016 Aug 10. Nanotoxicology. 2016. PMID: 27441588
-
Genotoxicity of polyvinylpyrrolidone-coated silver nanoparticles in BEAS 2B cells.Toxicology. 2013 Nov 8;313(1):38-48. doi: 10.1016/j.tox.2012.09.014. Epub 2012 Nov 8. Toxicology. 2013. PMID: 23142790
-
From the Cover: An Investigation of the Genotoxicity and Interference of Gold Nanoparticles in Commonly Used In Vitro Mutagenicity and Genotoxicity Assays.Toxicol Sci. 2017 Mar 1;156(1):149-166. doi: 10.1093/toxsci/kfw247. Toxicol Sci. 2017. PMID: 28108664
-
Silver Nanoparticle-Mediated Cellular Responses in Various Cell Lines: An in Vitro Model.Int J Mol Sci. 2016 Sep 22;17(10):1603. doi: 10.3390/ijms17101603. Int J Mol Sci. 2016. PMID: 27669221 Free PMC article. Review.
-
Genotoxicity of environmental agents assessed by the alkaline comet assay.Basic Clin Pharmacol Toxicol. 2005;96 Suppl 1:1-42. Basic Clin Pharmacol Toxicol. 2005. PMID: 15859009 Review.
Cited by
-
Novel Antibacterial, Cytotoxic and Catalytic Activities of Silver Nanoparticles Synthesized from Acidophilic Actinobacterial SL19 with Evidence for Protein as Coating Biomolecule.J Microbiol Biotechnol. 2022 Sep 28;32(9):1195-1208. doi: 10.4014/jmb.2205.05006. Epub 2022 Aug 22. J Microbiol Biotechnol. 2022. PMID: 36116918 Free PMC article.
-
Metal Oxide Nanoparticles in Food Packaging and Their Influence on Human Health.Foods. 2023 May 3;12(9):1882. doi: 10.3390/foods12091882. Foods. 2023. PMID: 37174420 Free PMC article. Review.
-
Diaminobenzidine Photooxidation to Visualize Fluorescent Nanoparticles in Adhering Cultured Cells at Transmission Electron Microscopy.Methods Mol Biol. 2023;2566:333-343. doi: 10.1007/978-1-0716-2675-7_27. Methods Mol Biol. 2023. PMID: 36152264
-
A Review of the Antibacterial, Fungicidal and Antiviral Properties of Selenium Nanoparticles.Materials (Basel). 2023 Jul 30;16(15):5363. doi: 10.3390/ma16155363. Materials (Basel). 2023. PMID: 37570068 Free PMC article. Review.
-
Green Synthesis of Ag-MnO2 Nanoparticles using Chelidonium majus and Vinca minor Extracts and Their In Vitro Cytotoxicity.Molecules. 2020 Feb 13;25(4):819. doi: 10.3390/molecules25040819. Molecules. 2020. PMID: 32070017 Free PMC article.
References
-
- Feng Q. L., Wu J., Chen G. Q., Cui F. Z., Kim T. N., Kim J. O. (2000) A mechanistic study of the antibacterial effect of silver ions on Escherichia coli and Staphylococcus aureus. J. Biomed. Mater. Res., 52, 662–668. - PubMed
-
- Kim J. Y., Lee C., Cho M., Yoon J. (2008) Enhanced inactivation of E. coli and MS-2 phage by silver ions combined with UV-A and visible light irradiation. Water Res., 42, 356–362. - PubMed
-
- Ghandour W., Hubbard J. A., Deistung J., Hughes M. N., Poole R. K. (1988) The uptake of silver ions by Escherichia-coli-K12 - toxic effects and interaction with copper ions. Appl. Microbiol. Biotechnol., 28, 559–565.
-
- Bragg P. D., Rainnie D. J. (1974) The effect of silver ions on the respiratory chain of Escherichia coli. Can. J. Microbiol., 20, 883–889. - PubMed
-
- Slawson R. M., Lohmeier-Vogel E. M., Lee H., Trevors J. T. (1994) Silver resistance in Pseudomonas stutzeri. Biometals, 7, 30–40. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

