Streptococcus pneumoniae triggers progression of pulmonary fibrosis through pneumolysin
- PMID: 25964315
- PMCID: PMC6729139
- DOI: 10.1136/thoraxjnl-2014-206420
Streptococcus pneumoniae triggers progression of pulmonary fibrosis through pneumolysin
Abstract
Rationale: Respiratory tract infections are common in patients suffering from pulmonary fibrosis. The interplay between bacterial infection and fibrosis is characterised poorly.
Objectives: To assess the effect of Gram-positive bacterial infection on fibrosis exacerbation in mice.
Methods: Fibrosis progression in response to Streptococcus pneumoniae was examined in two different mouse models of pulmonary fibrosis.
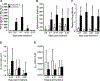
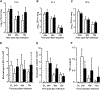
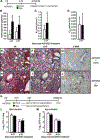
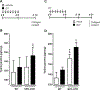
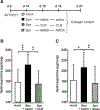
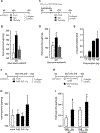
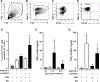
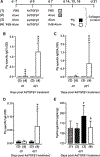
Measurements and main results: We demonstrate that wild-type mice exposed to adenoviral vector delivery of active transforming growth factor-β1 (TGFß1) or diphteria toxin (DT) treatment of transgenic mice expressing the DT receptor (DTR) under control of the surfactant protein C (SPC) promoter (SPC-DTR) to induce pulmonary fibrosis developed progressive fibrosis following infection with Spn, without exhibiting impaired lung protective immunity against Spn. Antibiotic treatment abolished infection-induced fibrosis progression. The cytotoxin pneumolysin (Ply) of Spn caused this phenomenon in a TLR4-independent manner, as Spn lacking Ply (SpnΔply) failed to trigger progressive fibrogenesis, whereas purified recombinant Ply did. Progressive fibrogenesis was also observed in AdTGFβ1-exposed Ply-challenged TLR4 KO mice. Increased apoptotic cell death of alveolar epithelial cells along with an attenuated intrapulmonary release of antifibrogenic prostaglandin E2 was found to underlie progressive fibrogenesis in Ply-challenged AdTGFβ1-exposed mice. Importantly, vaccination of mice with the non-cytotoxic Ply derivative B (PdB) substantially attenuated Ply-induced progression of lung fibrosis in AdTGFβ1-exposed mice.
Conclusions: Our data unravel a novel mechanism by which infection with Spn through Ply release induces progression of established lung fibrosis, which can be attenuated by protein-based vaccination of mice.
Keywords: Bacterial Infection; Idiopathic pulmonary fibrosis; Respiratory Infection.
Published by the BMJ Publishing Group Limited. For permission to use (where not already granted under a licence) please go to http://group.bmj.com/group/rights-licensing/permissions.
Figures
Similar articles
-
Acacetin inhibits Streptococcus pneumoniae virulence by targeting pneumolysin.J Pharm Pharmacol. 2020 Aug;72(8):1092-1100. doi: 10.1111/jphp.13279. Epub 2020 May 10. J Pharm Pharmacol. 2020. PMID: 32390150
-
Efferocytosis of apoptotic alveolar epithelial cells is sufficient to initiate lung fibrosis.Cell Death Dis. 2018 Oct 17;9(11):1056. doi: 10.1038/s41419-018-1074-z. Cell Death Dis. 2018. PMID: 30333529 Free PMC article.
-
Dual function of pneumolysin in the early pathogenesis of murine pneumococcal pneumonia.J Clin Invest. 1995 Jan;95(1):142-50. doi: 10.1172/JCI117631. J Clin Invest. 1995. PMID: 7814608 Free PMC article.
-
Pneumolysin: a multifunctional pneumococcal virulence factor.J Lab Clin Med. 1998 Jan;131(1):21-7. doi: 10.1016/s0022-2143(98)90073-7. J Lab Clin Med. 1998. PMID: 9452123 Review.
-
The biology of pneumolysin.Subcell Biochem. 2014;80:145-60. doi: 10.1007/978-94-017-8881-6_8. Subcell Biochem. 2014. PMID: 24798011 Review.
Cited by
-
The Epithelial-Immune Crosstalk in Pulmonary Fibrosis.Front Immunol. 2021 May 19;12:631235. doi: 10.3389/fimmu.2021.631235. eCollection 2021. Front Immunol. 2021. PMID: 34093523 Free PMC article. Review.
-
Repetitive invasive lung function maneuvers do not accentuate experimental fibrosis in mice.Sci Rep. 2024 Jun 14;14(1):13774. doi: 10.1038/s41598-024-64548-w. Sci Rep. 2024. PMID: 38877042 Free PMC article.
-
Epigallocatechin gallate inhibits Streptococcus pneumoniae virulence by simultaneously targeting pneumolysin and sortase A.J Cell Mol Med. 2017 Oct;21(10):2586-2598. doi: 10.1111/jcmm.13179. Epub 2017 Apr 12. J Cell Mol Med. 2017. PMID: 28402019 Free PMC article.
-
The immune mechanisms of acute exacerbations of idiopathic pulmonary fibrosis.Front Immunol. 2024 Dec 16;15:1450688. doi: 10.3389/fimmu.2024.1450688. eCollection 2024. Front Immunol. 2024. PMID: 39737178 Free PMC article. Review.
-
Commensal Oral Microbiota, Disease Severity, and Mortality in Fibrotic Lung Disease.Am J Respir Crit Care Med. 2024 May 1;209(9):1101-1110. doi: 10.1164/rccm.202308-1357OC. Am J Respir Crit Care Med. 2024. PMID: 38051927 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
