N-lactoyl-amino acids are ubiquitous metabolites that originate from CNDP2-mediated reverse proteolysis of lactate and amino acids
- PMID: 25964343
- PMCID: PMC4450436
- DOI: 10.1073/pnas.1424638112
N-lactoyl-amino acids are ubiquitous metabolites that originate from CNDP2-mediated reverse proteolysis of lactate and amino acids
Abstract
Despite technological advances in metabolomics, large parts of the human metabolome are still unexplored. In an untargeted metabolomics screen aiming to identify substrates of the orphan transporter ATP-binding cassette subfamily C member 5 (ABCC5), we identified a class of mammalian metabolites, N-lactoyl-amino acids. Using parallel protein fractionation in conjunction with shotgun proteomics on fractions containing N-lactoyl-Phe-forming activity, we unexpectedly found that a protease, cytosolic nonspecific dipeptidase 2 (CNDP2), catalyzes their formation. N-lactoyl-amino acids are ubiquitous pseudodipeptides of lactic acid and amino acids that are rapidly formed by reverse proteolysis, a process previously considered to be negligible in vivo. The plasma levels of these metabolites strongly correlate with plasma levels of lactate and amino acid, as shown by increased levels after physical exercise and in patients with phenylketonuria who suffer from elevated Phe levels. Our approach to identify unknown metabolites and their biosynthesis has general applicability in the further exploration of the human metabolome.
Keywords: ABCC5; MRP5; physical exercise; unknown metabolites; untargeted metabolomics.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

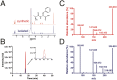

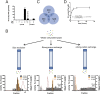
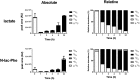

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

