Characterization of dexmedetomidine dosing and safety in neonates and infants
- PMID: 25964728
- PMCID: PMC4418678
- DOI: 10.5863/1551-6776-20.2.112
Characterization of dexmedetomidine dosing and safety in neonates and infants
Abstract
Objectives: To describe and compare off-label use and cardiovascular (CV) adverse effects of dexmedetomidine in neonates and infants in the pediatric intensive care unit (PICU).
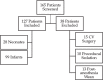
Methods: Patients younger than 12 months with corrected gestational ages of at least 37 weeks who were receiving continuous infusion of dexmedetomidine at a tertiary pediatric referral center between October 2007 and August 2012 were assessed retrospectively. Patients were excluded if dexmedetomidine was used for procedural sedation, postoperative CV surgery, or if postanesthesia infusion weaning orders existed at the time of PICU admission.
Results: The median minimum dexmedetomidine dose was similar between infants and neonates at 0.2 mcg/kg/hr (IQR, 0.17-0.3) versus 0.29 mcg/kg/hr (IQR, 0.2-0.31), p = 0.35. The median maximum dose was higher for infants than neonates (0.6 mcg/kg/hr [IQR, 0.4-0.8] vs. 0.4 mcg/kg/hr [IQR, 0.26-0.6], p < 0.01). Additional sedative use was more common in infants than neonates (75/99 [76%] vs. 15/28 [54%], p = 0.02). At least 1 episode of hypotension was noted in 34/127 (27%) patients and was similar between groups. An episode of bradycardia was identified more frequently in infants than neonates (55/99 [56%] vs. 2/28 [7%], p < 0.01). Significant reduction in heart rate and systolic blood pressure was noted when comparing baseline vital signs to lowest heart rate and systolic blood pressure during infusion (p < 0.01).
Conclusions: Dexmedetomidine dose ranges were similar to US Food and Drug Administration-labeled dosages for intensive care unit sedation in adults. More infants than neonates experienced a bradycardia episode, but infants were also more likely to receive higher dosages of dexmedetomidine and additional sedatives.
Keywords: adverse drug event; dexmedetomidine; infant; neonate.
Figures
References
-
- Tan JA, Ho KM. Use of dexmedetomidine as a sedative and analgesic agent in critically ill adult patients: a meta-analysis. Intensive Care Med. 2010;36(6):926–939. - PubMed
-
- Greenwood Village, CO: Thomson Micromedex; Dexmedetomidine. DrugPoint Summary. http://thomsonhc.com. Accessed October 12, 2014.
-
- Hudson, OH: Lexi-Comp, Inc; Dexmedetomidine. Lexi-Drugs Online. Lexi-Comp Online. http://online.lexi.com/crlonline. Accessed October 12, 2014.
-
- Phan H, Nahata MC. Clinical uses of dexmedetomidine in pediatric patients. Pediatr Drugs. 2008;10(1):49–69. - PubMed
-
- Su F, Hammer G. Dexmedetomidine: pediatric pharmacology, clinical uses and safety. Expert Opin Drug Saf. 2011;10(1):55–66. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources