Stim and Orai proteins in neuronal Ca(2+) signaling and excitability
- PMID: 25964739
- PMCID: PMC4408853
- DOI: 10.3389/fncel.2015.00153
Stim and Orai proteins in neuronal Ca(2+) signaling and excitability
Abstract
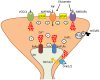
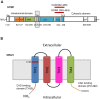
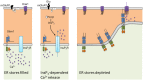
Stim1 and Orai1 are ubiquitous proteins that have long been known to mediate Ca(2+) release-activated Ca(2+) (CRAC) current (ICRAC) and store-operated Ca(2+) entry (SOCE) only in non-excitable cells. SOCE is activated following the depletion of the endogenous Ca(2+) stores, which are mainly located within the endoplasmic reticulum (ER), to replete the intracellular Ca(2+) reservoir and engage specific Ca(2+)-dependent processes, such as proliferation, migration, cytoskeletal remodeling, and gene expression. Their paralogs, Stim2, Orai2 and Orai3, support SOCE in heterologous expression systems, but their physiological role is still obscure. Ca(2+) inflow in neurons has long been exclusively ascribed to voltage-operated and receptor-operated channels. Nevertheless, recent work has unveiled that Stim1-2 and Orai1-2, but not Orai3, proteins are also expressed and mediate SOCE in neurons. Herein, we survey current knowledge about the neuronal distribution of Stim and Orai proteins in rodent and human brains; we further discuss that Orai2 is the main pore-forming subunit of CRAC channels in central neurons, in which it may be activated by either Stim1 or Stim2 depending on species, brain region and physiological stimuli. We examine the functions regulated by SOCE in neurons, where this pathway is activated under resting conditions to refill the ER, control spinogenesis and regulate gene transcription. Besides, we highlighted the possibility that SOCE also controls neuronal excitation and regulate synaptic plasticity. Finally, we evaluate the involvement of Stim and Orai proteins in severe neurodegenerative and neurological disorders, such as Alzheimer's disease and epilepsy.
Keywords: Ca2+ signaling; Orai1; Orai2; STIM1; STIM2; neurons; store-operated Ca2+ entry.
Figures
References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

