Topical, Aqueous, Clear Cyclosporine Formulation Design for Anterior and Posterior Ocular Delivery
- PMID: 25964868
- PMCID: PMC4418434
- DOI: 10.1167/tvst.4.3.1
Topical, Aqueous, Clear Cyclosporine Formulation Design for Anterior and Posterior Ocular Delivery
Abstract
Purpose: The main objective of this study was to optimize cyclosporine (CsA) nanomicellar solution and study in vivo ocular CsA tissue distribution with a topical drop.
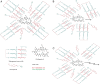
Methods: An optimized blend of hydrogenated castor oil-40 and octoxynol-40 was prepared to entrap CsA within nanomicelles. In vivo studies were conducted in New Zealand White albino rabbits with topical drop instillation.
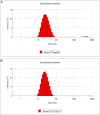
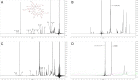
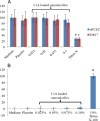
Results: Average size of CsA-loaded nanomicelles was approximately 22.4 nm. Ocular tissue CsA quantification with single and multiple dosing revealed that CsA levels followed as cornea → iris-ciliary body → aqueous humor → lens. Cyclosporine levels were also found to be in the following order: conjunctiva → sclera → retina/choroid → vitreous humor. High CsA level was detected in retina/choroid (53.7 ng/g tissue).
Conclusions: Ocular tissue CsA distribution studies revealed high CsA concentrations in anterior ocular tissues. Moreover, it appears that nanomicelles are transported through a conjunctival-scleral pathway and deliver CsA to the retina/choroid. Results suggest polymeric blend to be a safe carrier for anterior and posterior ocular tissues.
Translational relevance: This study has significant translational relevance, disclosing results that suggest that aqueous nanomicellar approach can provide high corneal and conjunctival CsA concentrations. Aqueous nanomicelles can deliver high drug concentrations not only to anterior but also to back of the eye tissues, including retina. This article provides a platform for noninvasive back of the eye drug delivery with topical eye drops. Aqueous CsA nanomicelles have no perceptible toxicity such as cell membrane damage or cytotoxicity to corneal and retinal pigment epithelial cells. Clear aqueous nanomicellar solution can be translated to human conditions for keratoconjunctivitis sicca and other anti-inflammatory conditions.
Keywords: back of the eye; cyclosporine; drops; drug delivery; dry eye; formulation; nanomicelles; posterior; rabbits; retina/choroid; sclera; topical.
Figures

References
-
- Mondon K,, Zeisser-Labouebe M, Gurny R,, et al. Novel cyclosporin A formulations using MPEG-hexyl-substituted polylactide micelles: a suitability study. Eur J Pharm Biopharm. 2011; 77: 56–65. - PubMed
-
- Matsuda S, Koyasu S.Mechanisms of action of cyclosporine. Immunopharmacology. 2000; 47: 119–125. - PubMed
-
- Whitcup SM, Chan CC,, Luyo DA, et al. Topical cyclosporine inhibits mast cell-mediated conjunctivitis. Invest Ophthalmol Vis Sci. 1996; 37: 2686–2693. - PubMed
-
- Kunert KS,, Tisdale AS, Gipson IK.Goblet cell numbers and epithelial proliferation in the conjunctiva of patients with dry eye syndrome treated with cyclosporine. Arch Ophthalmol. 2002; 120: 330–337. - PubMed
-
- Gunduz K, Ozdemir O.Topical cyclosporin treatment of keratoconjunctivitis sicca in secondary Sjogren's syndrome. Acta Ophthalmol (Copenh). 1994; 72: 438–442. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

