SP-R210 (Myo18A) Isoforms as Intrinsic Modulators of Macrophage Priming and Activation
- PMID: 25965346
- PMCID: PMC4428707
- DOI: 10.1371/journal.pone.0126576
SP-R210 (Myo18A) Isoforms as Intrinsic Modulators of Macrophage Priming and Activation
Abstract
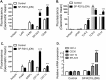
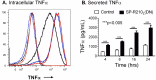
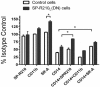
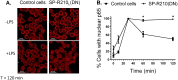
The surfactant protein (SP-A) receptor SP-R210 has been shown to increase phagocytosis of SP-A-bound pathogens and to modulate cytokine secretion by immune cells. SP-A plays an important role in pulmonary immunity by enhancing opsonization and clearance of pathogens and by modulating macrophage inflammatory responses. Alternative splicing of the Myo18A gene results in two isoforms: SP-R210S and SP-R210L, with the latter predominantly expressed in alveolar macrophages. In this study we show that SP-A is required for optimal expression of SP-R210L on alveolar macrophages. Interestingly, pre-treatment with SP-A prepared by different methods either enhances or suppresses responsiveness to LPS, possibly due to differential co-isolation of SP-B or other proteins. We also report that dominant negative disruption of SP-R210L augments expression of receptors including SR-A, CD14, and CD36, and enhances macrophages' inflammatory response to TLR stimulation. Finally, because SP-A is known to modulate CD14, we used a variety of techniques to investigate how SP-R210 mediates the effect of SP-A on CD14. These studies revealed a novel physical association between SP-R210S, CD14, and SR-A leading to an enhanced response to LPS, and found that SP-R210L and SP-R210S regulate internalization of CD14 via distinct macropinocytosis-like mechanisms. Together, our findings support a model in which SP-R210 isoforms differentially regulate trafficking, expression, and activation of innate immune receptors on macrophages.
Conflict of interest statement
Figures





Similar articles
-
SP-R210 isoforms of Myosin18A modulate endosomal sorting and recognition of influenza A virus infection in macrophages.Microbes Infect. 2024 Mar-Apr;26(3):105280. doi: 10.1016/j.micinf.2023.105280. Epub 2023 Dec 21. Microbes Infect. 2024. PMID: 38135024 Free PMC article.
-
Genomic and epigenomic adaptation in SP-R210 (Myo18A) isoform-deficient macrophages.Immunobiology. 2021 Nov;226(6):152150. doi: 10.1016/j.imbio.2021.152150. Epub 2021 Oct 25. Immunobiology. 2021. PMID: 34735924 Free PMC article.
-
Surfactant protein A (SP-A)-mediated clearance of Staphylococcus aureus involves binding of SP-A to the staphylococcal adhesin eap and the macrophage receptors SP-A receptor 210 and scavenger receptor class A.J Biol Chem. 2011 Feb 11;286(6):4854-70. doi: 10.1074/jbc.M110.125567. Epub 2010 Dec 1. J Biol Chem. 2011. PMID: 21123169 Free PMC article.
-
Immunoregulatory function of SP-A.Mol Immunol. 2024 Feb;166:58-64. doi: 10.1016/j.molimm.2024.01.005. Epub 2024 Jan 19. Mol Immunol. 2024. PMID: 38244369 Review.
-
The Role of Collectins and Galectins in Lung Innate Immune Defense.Front Immunol. 2018 Sep 4;9:1998. doi: 10.3389/fimmu.2018.01998. eCollection 2018. Front Immunol. 2018. PMID: 30233589 Free PMC article. Review.
Cited by
-
Surfactant protein A alters endosomal trafficking of influenza A virus in macrophages.Front Immunol. 2023 Mar 7;14:919800. doi: 10.3389/fimmu.2023.919800. eCollection 2023. Front Immunol. 2023. PMID: 36960051 Free PMC article.
-
From Anti-SARS-CoV-2 Immune Response to the Cytokine Storm via Molecular Mimicry.Antibodies (Basel). 2021 Sep 24;10(4):36. doi: 10.3390/antib10040036. Antibodies (Basel). 2021. PMID: 34698069 Free PMC article.
-
Structure, genetics and function of the pulmonary associated surfactant proteins A and D: The extra-pulmonary role of these C type lectins.Ann Anat. 2017 May;211:184-201. doi: 10.1016/j.aanat.2017.03.002. Epub 2017 Mar 27. Ann Anat. 2017. PMID: 28351530 Free PMC article. Review.
-
SP-R210 isoforms of Myosin18A modulate endosomal sorting and recognition of influenza A virus infection in macrophages.Microbes Infect. 2024 Mar-Apr;26(3):105280. doi: 10.1016/j.micinf.2023.105280. Epub 2023 Dec 21. Microbes Infect. 2024. PMID: 38135024 Free PMC article.
-
Genomic and epigenomic adaptation in SP-R210 (Myo18A) isoform-deficient macrophages.Immunobiology. 2021 Nov;226(6):152150. doi: 10.1016/j.imbio.2021.152150. Epub 2021 Oct 25. Immunobiology. 2021. PMID: 34735924 Free PMC article.
References
-
- Mori K, Furusawa T, Okubo T, Inoue T, Ikawa S, Yanai N, et al. Genome structure and differential expression of two isoforms of a novel PDZ-containing myosin (MysPDZ) (Myo18A). Journal of biochemistry. 2003;133(4):405–13. Epub 2003/05/23. . - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

