Use of Fixed Dose Combination (FDC) Drugs in India: Central Regulatory Approval and Sales of FDCs Containing Non-Steroidal Anti-Inflammatory Drugs (NSAIDs), Metformin, or Psychotropic Drugs
- PMID: 25965416
- PMCID: PMC4428752
- DOI: 10.1371/journal.pmed.1001826
Use of Fixed Dose Combination (FDC) Drugs in India: Central Regulatory Approval and Sales of FDCs Containing Non-Steroidal Anti-Inflammatory Drugs (NSAIDs), Metformin, or Psychotropic Drugs
Abstract
Background: In 2012, an Indian parliamentary committee reported that manufacturing licenses for large numbers of fixed dose combination (FDC) drugs had been issued by state authorities without prior approval of the Central Drugs Standard Control Organization (CDSCO) in violation of rules, and considered that some ambiguity until 1 May 2002 about states' powers might have contributed. To our knowledge, no systematic enquiry has been undertaken to determine if evidence existed to support these findings. We investigated CDSCO approvals for and availability of oral FDC drugs in four therapeutic areas: analgesia (non-steroidal anti-inflammatory drugs [NSAIDs]), diabetes (metformin), depression/anxiety (anti-depressants/benzodiazepines), and psychosis (anti-psychotics).
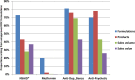
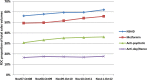
Methods and findings: This was an ecologic study with a time-trend analysis of FDC sales volumes (2007-2012) and a cross-sectional examination of 2011-2012 data to establish the numbers of formulations on the market with and without a record of CDSCO approval ("approved" and "unapproved"), their branded products, and sales volumes. Data from the CDSCO on approved FDC formulations were compared with sales data from PharmaTrac, a database of national drug sales. We determined the proportions of FDC sales volumes (2011-2012) arising from centrally approved and unapproved formulations and from formulations including drugs banned/restricted internationally. We also determined the proportions of centrally approved and unapproved formulations marketed before and after 1 May 2002, when amendments were made to the drug rules. FDC approvals in India, the United Kingdom (UK), and United States of America (US) were compared. For NSAID FDCs, 124 formulations were marketed, of which 34 (27%) were centrally approved and 90 (73%) were unapproved; metformin: 25 formulations, 20 (80%) approved, five (20%) unapproved; anti-depressants/benzodiazepines: 16 formulations, three (19%) approved, 13 (81%) unapproved; anti-psychotics: ten formulations, three (30%) approved, seven (70%) unapproved. After 1 May 2002, the proportions of approved FDC formulations increased for NSAIDs (26%/28%) and anti-psychotics (0%/38%) and decreased for metformin (100%/75%) and anti-depressants/benzodiazepines (20%/18%), and the overall proportion approved remained similar before and after that date. FDC formulations gave rise to multiple branded products, ranging from 211 anti-psychotic FDC products from ten formulations to 2,739 NSAID FDC products from 124 formulations. The proportions of FDC sales volumes arising from unapproved formulations were as follows: anti-depressants/benzodiazepines, 69%; anti-psychotics, 43%; NSAIDs, 28%; and metformin, 0.4%. Formulations including drugs banned/restricted internationally comprised over 12% of NSAID FDC sales and 53% of anti-psychotic FDC sales. Across the four therapeutic areas, 14 FDC formulations were approved in the UK and 22 in the US.
Conclusions: There was evidence supporting concerns about FDCs. Metformin excepted, substantial numbers of centrally unapproved formulations for NSAID, anti-depressant/benzodiazepine, and anti-psychotic FDCs were marketed; sales volumes were high. The legal need for central approval of new drugs before manufacture has been in place continuously since 1961, including for FDCs meeting the applicable legal test. Proportions of centrally unapproved formulations after 1 May 2002 did not decrease overall, and no ambiguity was found about states' licensing powers. Unapproved formulations should be banned immediately, prioritising those withdrawn/banned internationally and undertaking a review of benefits and risks for patients in ceasing or switching to other medicines. Drug laws need to be amended to ensure the safety and effectiveness of medicines marketed in India.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
References
-
- Harris G (2014 Apr 14) Medicines made in India set off safety worries. New York Times. http://www.nytimes.com/2014/02/15/world/asia/medicines-made-in-india-set.... Accessed 30 March 2015.
-
- European Medicines Agency (2015 Jan 23) GVK Biosciences: European Medicines Agency recommends suspending medicines over flawed studies. http://www.ema.europa.eu/ema/index.jsp?curl=pages/news_and_events/news/2.... Accessed 30 March 2015.
-
- Porecha M (2014 Apr 22) Vietnam shows the door to 45 Indian pharma companies. DNA (Diligent Media Corporation). http://www.dnaindia.com/mumbai/report-vietnam-shows-the-door-to-45-india.... Accessed 30 March 2015.
-
- Ministry of Health and Family Welfare (2003) Report of the expert committee on a comprehensive examination of drug regulatory issues, including the problem of spurious drugs. http://planningcommission.nic.in/reports/genrep/health/Final_Report_mash.... Accessed 30 March 2015.
-
- Community Development Medicinal Unit, Health Action International Asia Pacific (2010 Dec 15) Report on research project: ‘fixed-dose combination in India, inception—marketing—a study’. http://www.cdmuindia.org/Pdf-files/Final-report-IFDCs.pdf. Accessed 30 March 2015.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

