Genotype-Associated Differential NKG2D Expression on CD56+CD3+ Lymphocytes Predicts Response to Pegylated-Interferon/Ribavirin Therapy in Chronic Hepatitis C
- PMID: 25965701
- PMCID: PMC4428701
- DOI: 10.1371/journal.pone.0125664
Genotype-Associated Differential NKG2D Expression on CD56+CD3+ Lymphocytes Predicts Response to Pegylated-Interferon/Ribavirin Therapy in Chronic Hepatitis C
Abstract
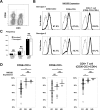
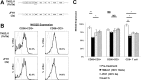
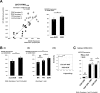
Hepatitis C virus (HCV) genotype 1 infections are significantly more difficult to eradicate with PEG-IFN/ribavirin therapy, compared to HCV genotype 2. The aim of this work is to investigate the difference of immunological impairments underlying this phenomenon. Pre-treatment NKG2D expression on peripheral CD56+CD3+ lymphocytes and CD56+CD3- NK cells from cases of chronic hepatitis C were analyzed and assessed by treatment effect. Two strains of HCV were used to co-incubate with immune cells in vitro. NKG2D expression on peripheral CD56+CD3+ lymphocytes, but not NK cells, was significantly impaired in genotype 1 infection, compared to genotype 2. When peripheral blood mononuclear cells from healthy donors were co-incubated with TNS2J1, a genotype 1b/2a chimera strain, or with JFH1, a genotype 2a strain, genotype-specific decrease of NKG2D on CD56+CD3+ lymphocytes, but not NK cells, was observed. Pre-treatment NKG2D expression on peripheral CD56+CD3+ lymphocytes significantly correlated with reduction in serum HCV RNA levels from week 0 to week 4, and predicted treatment response. Ex vivo stimulation of peripheral CD56+CD3+ lymphocytes showed NKG2D expression-correlated IFN-γ production. In conclusion, Decreased NKG2D expression on CD56+CD3+ lymphocytes in chronic HCV genotype 1 infection predicts inferior treatment response to PEG-IFN/ribavirin therapy compared to genotype 2.
Conflict of interest statement
Figures
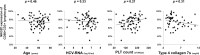

References
-
- Ebinuma H, Nakamoto N, Li Y, Price DA, Gostick E, Levine BL, et al. Identification and in vitro expansion of functional antigen-specific CD25+ FoxP3+ regulatory T cells in hepatitis C virus infection. Journal of virology. 2008;82(10):5043–53. Epub 2008/03/14. 10.1128/JVI.01548-07 PubMed - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

