BMP-2 Is Involved in Scleral Remodeling in Myopia Development
- PMID: 25965995
- PMCID: PMC4429026
- DOI: 10.1371/journal.pone.0125219
BMP-2 Is Involved in Scleral Remodeling in Myopia Development
Abstract
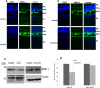
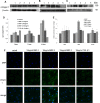
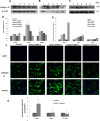
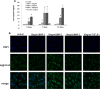
The development of myopia is associated with scleral remodeling, but it is unclear which factors regulate this process. This study investigated bone morphogenetic protein-2 (BMP-2) expression in the sclera of guinea pigs with lens-induced myopia (LIM) and after recovery from myopia and evaluated the effect of BMP-2 on extracellular matrix (ECM) synthesis in human scleral fibroblasts (HSFs) cultured in vitro. Lens-induced myopia was brought about in two groups of guinea pigs (the lens-induced myopia and myopia recovery groups) by placing -4.00 D lenses on the right eye for three weeks. The left eye served as a contralateral control. In the recovery group, the lenses were removed after one week. The refractive power and axial length of the eyes were measured, and the BMP-2 expression levels in the sclera were measured. After three weeks, the lens-induced eyes acquired relative myopia in both groups of guinea pigs. Immunostaining of the eyeballs revealed significantly decreased BMP-2 expression in the posterior sclera of the myopic eyes compared to the contralateral eyes. One week after lens removal, BMP-2 expression recovered, and no differences were observed between the experimental and contralateral eyes in the recovery group. HSFs were cultured with BMP-2 or transforming growth factor-β1 (TGF-β1). Type I and type III collagen synthesis was significantly up-regulated following BMP-2 treatment in culture after one and two weeks, but the ratio of type III to type I collagen mRNA was not increased. Biosynthesis of glycosaminoglycan (GAG) and aggrecan was increased in HSFs treated with BMP-2. Some chondrogenesis-associated genes expression increased in HSFs treated with BMP-2. From this study, we concluded that BMP-2 is involved in scleral remodeling in the development and recovery of lens-induced myopia.
Conflict of interest statement
Figures

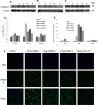
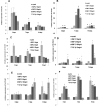
References
-
- Lin LL, Shih YF, Tsai CB, Chen CJ, Lee LA, Hung PT, et al. Epidemiologic study of ocular refraction among schoolchildren in Taiwan in 1995. 1999; Optom Vis Sci 76: 275–281. - PubMed
-
- Goh WS, Lam CS. Changes in refractive trends and optical components of Hong Kong Chinese aged 19–39 years. 1994; Ophthalmic Physiol Opt 14: 378–382. - PubMed
-
- Sperduto RD, Seigel D, Roberts J, Rowland M. Prevalence of myopia in the United States. 1983; Arch Ophthalmol 101: 405–407. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

