Endothelin receptors and their antagonists
- PMID: 25966344
- PMCID: PMC4437774
- DOI: 10.1016/j.semnephrol.2015.02.002
Endothelin receptors and their antagonists
Abstract
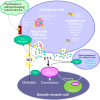
All three members of the endothelin (ET) family of peptides, ET-1, ET-2, and ET-3, are expressed in the human kidney, with ET-1 being the predominant isoform. ET-1 and ET-2 bind to two G-protein-coupled receptors, ETA and ETB, whereas at physiological concentrations ET-3 has little affinity for the ET(A) receptor. The human kidney is unusual among the peripheral organs in expressing a high density of ET(B). The renal vascular endothelium only expresses the ET(B) subtype and ET-1 acts in an autocrine or paracrine manner to release vasodilators. Endothelial ETB in kidney, as well as liver and lungs, also has a critical role in scavenging ET-1 from the plasma. The third major function is ET-1 activation of ET(B) in in the nephron to reduce salt and water re-absorption. In contrast, ET(A) predominate on smooth muscle, causing vasoconstriction and mediating many of the pathophysiological actions of ET-1. The role of the two receptors has been delineated using highly selective ET(A) (BQ123, TAK-044) and ET(B) (BQ788) peptide antagonists. Nonpeptide antagonists, bosentan, macitentan, and ambrisentan, that are either mixed ET(A)/ET(B) antagonists or display ET(A) selectivity, have been approved for clinical use but to date are limited to pulmonary hypertension. Ambrisentan is in clinical trials in patients with type 2 diabetic nephropathy. This review summarizes ET-receptor antagonism in the human kidney, and considers the relative merits of selective versus nonselective antagonism in renal disease.
Keywords: Ambrisentan; antagonist; bosentan; endothelin-1; macitentan; sitaxentan.
Copyright © 2015 The Authors. Published by Elsevier Inc. All rights reserved.
Figures

References
-
- Yanagisawa M., Kurihara H., Kimura S., Tomobe Y., Kobayashi M., Mitsui Y. A novel potent vasoconstrictor peptide produced by vascular endothelial cells. Nature. 1988;332:411–415. - PubMed
-
- Karet F.E., Davenport A.P. Localization of endothelin peptides in human kidney. Kidney Int. 1996;49:382–387. - PubMed
-
- Arai H., Hori S., Aramori I., Ohkubo H., Nakanishi S. Cloning and expression of a cDNA encoding an endothelin receptor. Nature. 1990;348:730–732. - PubMed
-
- Sakurai T., Yanagisawa M., Takuwa Y., Miyazaki H., Kimura S., Goto K. Cloning of a cDNA encoding a non-isopeptide-selective subtype of the endothelin receptor. Nature. 1990;348:732–735. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

