Endothelin and the renal microcirculation
- PMID: 25966346
- PMCID: PMC5221560
- DOI: 10.1016/j.semnephrol.2015.02.004
Endothelin and the renal microcirculation
Abstract
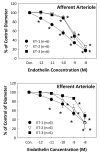
Endothelin (ET) is one of the most potent renal vasoconstrictors. Endothelin plays an essential role in the regulation of renal blood flow, glomerular filtration, sodium and water transport, and acid-base balance. ET-1, ET-2, and ET-3 are the three distinct endothelin isoforms comprising the endothelin family. ET-1 is the major physiologically relevant peptide and exerts its biological activity through two G-protein-coupled receptors: ET(A) and ET(B). Both ET(A) and ET(B) are expressed by the renal vasculature. Although ET(A) are expressed mainly by vascular smooth muscle cells, ET(B) are expressed by both renal endothelial and vascular smooth muscle cells. Activation of the endothelin system, or overexpression of downstream endothelin signaling pathways, has been implicated in several pathophysiological conditions including hypertension, acute kidney injury, diabetic nephropathy, and immune nephritis. In this review, we focus on the effects of endothelin on the renal microvasculature, and update recent findings on endothelin in the regulation of renal hemodynamics.
Keywords: Kidney; afferent arteriole; autoregulation; efferent arteriole; glomerular filtration.
Copyright © 2015 Elsevier Inc. All rights reserved.
Figures


References
-
- Hickey KA, Rubanyi G, Paul RJ, Highsmith RF. Characterization of a coronary vasoconstrictor produced by cultured endothelial cells. Am J Physiol. 1985;248(5 Pt 1):C550–6. - PubMed
-
- Yanagisawa M, Kurihara H, Kimura S, Tomobe Y, Kobayashi M, Mitsui Y, Yazaki Y, Goto K, Masaki T. A novel potent vasoconstrictor peptide produced by vascular endothelial cells. Nature. 1988;332(6163):411–5. - PubMed
-
- Itoh Y, Yanagisawa M, Ohkubo S, Kimura C, Kosaka T, Inoue A, Ishida N, Mitsui Y, Onda H, Fujino M, et al. Cloning and sequence analysis of cDNA encoding the precursor of a human endothelium-derived vasoconstrictor peptide, endothelin: identity of human and porcine endothelin. FEBS Lett. 1988;231(2):440–4. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

