A method for human teratogen detection by geometrically confined cell differentiation and migration
- PMID: 25966467
- PMCID: PMC4428054
- DOI: 10.1038/srep10038
A method for human teratogen detection by geometrically confined cell differentiation and migration
Erratum in
-
Corrigendum: A method for human teratogen detection by geometrically confined cell differentiation and migration.Sci Rep. 2015 Aug 3;5:12387. doi: 10.1038/srep12387. Sci Rep. 2015. PMID: 26237348 Free PMC article. No abstract available.
Abstract
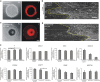
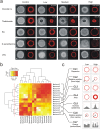
Unintended exposure to teratogenic compounds can lead to various birth defects; however current animal-based testing is limited by time, cost and high inter-species variability. Here, we developed a human-relevant in vitro model, which recapitulated two cellular events characteristic of embryogenesis, to identify potentially teratogenic compounds. We spatially directed mesoendoderm differentiation, epithelial-mesenchymal transition and the ensuing cell migration in micropatterned human pluripotent stem cell (hPSC) colonies to collectively form an annular mesoendoderm pattern. Teratogens could disrupt the two cellular processes to alter the morphology of the mesoendoderm pattern. Image processing and statistical algorithms were developed to quantify and classify the compounds' teratogenic potential. We not only could measure dose-dependent effects but also correctly classify species-specific drug (Thalidomide) and false negative drug (D-penicillamine) in the conventional mouse embryonic stem cell test. This model offers a scalable screening platform to mitigate the risks of teratogen exposures in human.
Figures



References
-
- Haschek W. M., Rousseaux C. G. & Wallig M. A. [Chapter 20 Developmental Pathology] Fundamentals of Toxicologic Pathology [634] Academic Press London, UK 2010).
-
- Bremer S., Pellizzer C., Hoffmann S., Seidle T. & Hartung T. The development of new concepts for assessing reproductive toxicity applicable to large scale toxicological programmes. Curr. Pharm. Des. 13, 3047–58 (2007). - PubMed
-
- Kameoka S., Babiarz J., Kolaja K. & Chiao E. A high-throughput screen for teratogens using human pluripotent stem cells. Toxicol. Sci. 137, 76–90 (2014). - PubMed
-
- Colleoni S. et al. Characterisation of a neural teratogenicity assay based on human ESCs differentiation following exposure to valproic acid. Curr. Med. Chem. 19, 6065–71 (2012). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

