Pharmacology of L-type Calcium Channels: Novel Drugs for Old Targets?
- PMID: 25966690
- PMCID: PMC5384371
- DOI: 10.2174/1874467208666150507105845
Pharmacology of L-type Calcium Channels: Novel Drugs for Old Targets?
Abstract
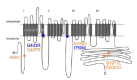
Inhibition of voltage-gated L-type calcium channels by organic calcium channel blockers is a well-established pharmacodynamic concept for the treatment of hypertension and cardiac ischemia. Since decades these antihypertensives (such as the dihydropyridines amlodipine, felodipine or nifedipine) belong to the most widely prescribed drugs world-wide. Their tolerability is excellent because at therapeutic doses their pharmacological effects in humans are limited to the cardiovascular system. During the last years substantial progress has been made to reveal the physiological role of different L-type calcium channel isoforms in many other tissues, including the brain, endocrine and sensory cells. Moreover, there is accumulating evidence about their involvement in various human diseases, such as Parkinson's disease, neuropsychiatric disorders and hyperaldosteronism. In this review we discuss the pathogenetic role of L-type calcium channels, potential new indications for existing or isoform-selective compounds and strategies to minimize potential side effects.
Figures


References
-
- Catterall W.A., Perez-Reyes E., Snutch T.P., Striessnig J. International Union of Pharmacology. XLVIII. Nomenclature and structure-function relationships of voltage-gated calcium channels. Pharmacol. Rev. 2005;57(4):411–425. - PubMed
-
- Simms B.A., Zamponi G.W. Neuronal voltage-gated calcium channels: structure, function, and dysfunction. Neuron. 2014;82(1):24–45. - PubMed
-
- Dolphin A.C. The α2δ subunits of voltage-gated calcium channels. Biochim. Biophys. Acta. 2013;1828(7):1541–1549. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

