Regulation of Cardiac Calcium Channels in the Fight-or-Flight Response
- PMID: 25966697
- PMCID: PMC4664455
- DOI: 10.2174/1874467208666150507103417
Regulation of Cardiac Calcium Channels in the Fight-or-Flight Response
Abstract
Intracellular calcium transients generated by activation of voltage-gated calcium (CaV) channels generate local signals, which initiate physiological processes such as secretion, synaptic transmission, and excitation-contraction coupling. Regulation of calcium entry through CaV channels is crucial for control of these physiological processes. In this article, I review experimental results that have emerged over several years showing that cardiac CaV1.2 channels form a local signaling complex, in which their proteolytically processed distal C-terminal domain, an A-Kinase Anchoring Protein, and cyclic AMP-dependent protein kinase (PKA) interact directly with the transmembrane core of the ion channel through the proximal C-terminal domain. This signaling complex is the substrate for β-adrenergic up-regulation of the CaV1.2 channel in the heart during the fight-or-flight response. Protein phosphorylation of two sites at the interface between the distal and proximal C-terminal domains contributes importantly to control of basal CaV1.2 channel activity, and phosphorylation of Ser1700 by PKA at that interface up-regulates CaV1.2 activity in response to β-adrenergic signaling. Thus, the intracellular C-terminal domain of CaV1.2 channels serves as a signaling platform, mediating beat-to-beat physiological regulation of channel activity and up-regulation by β-adrenergic signaling in the fight-or-flight response.
Conflict of interest statement
The author confirms that this article content has no conflict of interest.
Figures
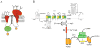
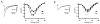
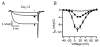

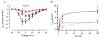

References
-
- Reuter H. Properties of two inward membrane currents in the heart. Annu Rev Physiol. 1979;41:413–424. - PubMed
-
- Tsien RW. Calcium channels in excitable cell membranes. Annu Rev Physiol. 1983;45:341–358. - PubMed
-
- Bers DM. Cardiac excitation-contraction coupling. Nature. 2002;415:198–205. - PubMed
-
- Reuter H. Calcium channel modulation by neurotransmitters, enzymes and drugs. Nature. 1983;301:569–574. - PubMed
-
- Catterall WA. Excitation-contraction coupling in vertebrate skeletal muscle: A tale of two calcium channels. Cell. 1991;64:871–874. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

