Stored red blood cell susceptibility to in vitro transfusion-associated stress conditions is higher after longer storage and increased by storage in saline-adenine-glucose-mannitol compared to AS-1
- PMID: 25968419
- PMCID: PMC4573361
- DOI: 10.1111/trf.13138
Stored red blood cell susceptibility to in vitro transfusion-associated stress conditions is higher after longer storage and increased by storage in saline-adenine-glucose-mannitol compared to AS-1
Abstract
Background: Biochemical changes induced in red blood cells (RBCs) during storage may impair their function upon transfusion. Transfusion-associated stresses may further amplify storage lesion effects including increased phosphatidylserine (PS) exposure at the RBC membrane, microparticle (MP) release, and adhesion to endothelial cells (ECs). RBC stress susceptibility in vitro was investigated in relation to storage time and additive solution.
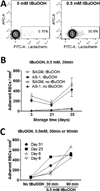
Study design and methods: Leukoreduced whole blood donations (n = 18) were paired, mixed, and resplit before separating the RBCs for storage in saline-adenine-glucose-mannitol (SAGM) or AS-1. Samples were taken after 3, 21, or 35 days. For oxidative stress treatment, RBCs were exposed to 0.5 mmol/L tert-butylhydroperoxide. Transfusion-associated stress was simulated by overnight culture at 37 °C with plasma containing inflammatory mediators. PS exposure and MPs were measured by flow cytometry and adhesion to ECs was tested under flow conditions. PS specificity of adhesion was tested by blocking with PS-containing lipid vesicles.
Results: Oxidative stress induced significantly higher PS exposure and adhesion to ECs in RBCs stored for 35 days compared to 3 days (p < 0.04). PS-containing vesicles blocked RBC-EC adhesion. After overnight culture with or without plasma, PS exposure and EC adhesion were significantly increased (p < 0.05). MP numbers increased with longer RBC storage and after RBC culture with plasma. Culture conditions influenced MP numbers from Day 35 RBCs. RBCs stored in SAGM had significantly higher PS exposure after stress treatment than AS-1 RBCs (p < 0.02).
Conclusion: Storage for 35 days significantly increased RBC susceptibility to oxidative and in vitro transfusion-associated stresses and was higher for RBCs stored in SAGM compared to AS-1.
© 2015 AABB.
Conflict of interest statement
There are no conflicts of interest.
Figures




References
-
- D'Alessandro A, Kriebardis AG, Rinalducci S, Antonelou MH, Hansen KC, Papassideri IS, et al. An update on red blood cell storage lesions, as gleaned through biochemistry and omics technologies. Transfusion. 2014 Aug 6; [Epub ahead of print] - PubMed
-
- Hess JR. Red cell storage. J Proteomics. 2010;73:368–373. - PubMed
-
- Acker JP, Hansen AL, Kurach JD, Turner TR, Croteau I, Jenkins C. A quality monitoring program for red blood cell components: in vitro quality indicators before and after implementation of semiautomated processing. Transfusion. 2014;54:2534–2543. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

