Preexisting Levels of CD4 T Cells Expressing PD-1 Are Related to Overall Survival in Prostate Cancer Patients Treated with Ipilimumab
- PMID: 25968455
- PMCID: PMC4561203
- DOI: 10.1158/2326-6066.CIR-14-0227
Preexisting Levels of CD4 T Cells Expressing PD-1 Are Related to Overall Survival in Prostate Cancer Patients Treated with Ipilimumab
Abstract
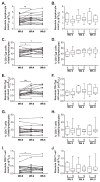
Cytotoxic T-lymphocyte-associated antigen-4 (CTLA-4) blockade can induce tumor regression and improved survival in cancer patients. This treatment can enhance adaptive immune responses without an exogenous vaccine, but the immunologic biomarkers associated with improved clinical outcome in cancer patients are not fully established. A phase Ib trial in patients with metastatic, castration-resistant prostate cancer was performed combining ipilimumab with sargramostim (GM-CSF). In addition to evaluating ipilimumab dose, patients were followed clinically for response and overall survival, and for immunomodulation of circulating T cells. PSA declines of ≥50% and radiographic responses were observed at doses of ≥3 mg/kg/dose. Timing of clinical responses could be either immediate or delayed. Durable responses were also observed off treatment. A subset of patients experienced long-term survival with or without objective clinical responses. The relationship between T-cell phenotype in peripheral blood and overall survival was examined retrospectively. We found that the treatment induced an increase in the levels of CD4(+) effector T (Teff) cells, regulatory T cells, PD-1(+) CD4 Teff cells, and PD-1(+) CD8 T cells. However, these increased levels were not associated with overall survival. Instead, low pretreatment baseline levels of PD-1(+) CD4 Teff cells were found to correlate with longer overall survival. Furthermore, baseline levels of PD-1(+) CD4 Teff cells from patients with shorter overall survival were higher than from cancer-free male control subjects. These results suggest that preexisting expression of immunologic checkpoint marker PD-1 on CD4 Teff cells may help identify patients that may benefit from ipilimumab treatment.
©2015 American Association for Cancer Research.
Conflict of interest statement
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

