Effects of aging, exercise, and disease on force transfer in skeletal muscle
- PMID: 25968577
- PMCID: PMC4490334
- DOI: 10.1152/ajpendo.00095.2015
Effects of aging, exercise, and disease on force transfer in skeletal muscle
Abstract
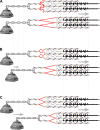
The loss of muscle strength and increased injury rate in aging skeletal muscle has previously been attributed to loss of muscle protein (cross-sectional area) and/or decreased neural activation. However, it is becoming clear that force transfer within and between fibers plays a significant role in this process as well. Force transfer involves a secondary matrix of proteins that align and transmit the force produced by the thick and thin filaments along muscle fibers and out to the extracellular matrix. These specialized networks of cytoskeletal proteins aid in passing force through the muscle and also serve to protect individual fibers from injury. This review discusses the cytoskeleton proteins that have been identified as playing a role in muscle force transmission, both longitudinally and laterally, and where possible highlights how disease, aging, and exercise influence the expression and function of these proteins.
Keywords: aging; dystrophin-glycoprotein complex; force transmission; injury.
Copyright © 2015 the American Physiological Society.
Figures


References
-
- Arber S, Halder G, Caroni P. Muscle LIM protein, a novel essential regulator of myogenesis, promotes myogenic differentiation. Cell 79: 221–231, 1994. - PubMed
-
- Barash IA, Bang ML, Mathew L, Greaser ML, Chen J, Lieber RL. Structural and regulatory roles of muscle ankyrin repeat protein family in skeletal muscle. Am J Physiol Cell Physiol 293: C218–C227, 2007. - PubMed
-
- Barash IA, Mathew L, Lahey M, Greaser ML, Lieber RL. Muscle LIM protein plays both structural and functional roles in skeletal muscle. Am J Physiol Cell Physiol 289: C1312–C1320, 2005. - PubMed
-
- Barash IA, Mathew L, Ryan AF, Chen J, Lieber RL. Rapid muscle-specific gene expression changes after a single bout of eccentric contractions in the mouse. Am J Physiol Cell Physiol 286: C355–C364, 2004. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

