Survival of skin cancer stem cells requires the Ezh2 polycomb group protein
- PMID: 25969142
- PMCID: PMC4580538
- DOI: 10.1093/carcin/bgv064
Survival of skin cancer stem cells requires the Ezh2 polycomb group protein
Abstract
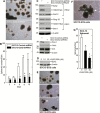
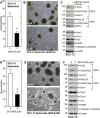
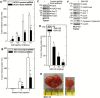
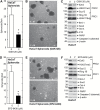
Polycomb group proteins, including Ezh2, are important candidate stem cell maintenance proteins in epidermal squamous cell carcinoma. We previously showed that epidermal cancer stem cells (ECS cells) represent a minority of cells in tumors, are highly enriched in Ezh2 and drive aggressive tumor formation. We now show that Ezh2 is required for ECS cell survival, migration, invasion and tumor formation and that this is associated with increased histone H3 trimethylation on lysine 27, a mark of Ezh2 action. We also show that Ezh2 knockdown or treatment with Ezh2 inhibitors, GSK126 or EPZ-6438, reduces Ezh2 level and activity, leading to reduced ECS cell spheroid formation, migration, invasion and tumor growth. These studies indicate that epidermal squamous cell carcinoma cells contain a subpopulation of cancer stem (tumor-initiating) cells that are enriched in Ezh2, that Ezh2 is required for optimal ECS cell survival and tumor formation and that treatment with Ezh2 inhibitors may be a strategy for reducing ECS cell survival and suppressing tumor formation.
© The Author 2015. Published by Oxford University Press. All rights reserved. For Permissions, please email: journals.permissions@oup.com.
Figures


References
-
- Gupta S., et al. (2002) Chemoprevention of skin cancer: current status and future prospects. Cancer Metastasis Rev., 21, 363–380. - PubMed
-
- Alam M., et al. (2001) Cutaneous squamous-cell carcinoma. N. Engl. J. Med., 344, 975–983. - PubMed
-
- Pincelli C., et al. (2010) Keratinocyte stem cells: friends and foes. J. Cell. Physiol., 225, 310–315. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

