Toward production of jet fuel functionality in oilseeds: identification of FatB acyl-acyl carrier protein thioesterases and evaluation of combinatorial expression strategies in Camelina seeds
- PMID: 25969557
- PMCID: PMC4493788
- DOI: 10.1093/jxb/erv225
Toward production of jet fuel functionality in oilseeds: identification of FatB acyl-acyl carrier protein thioesterases and evaluation of combinatorial expression strategies in Camelina seeds
Abstract
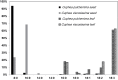
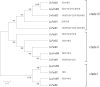
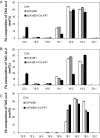
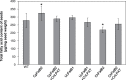
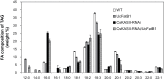
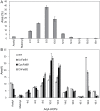
Seeds of members of the genus Cuphea accumulate medium-chain fatty acids (MCFAs; 8:0-14:0). MCFA- and palmitic acid- (16:0) rich vegetable oils have received attention for jet fuel production, given their similarity in chain length to Jet A fuel hydrocarbons. Studies were conducted to test genes, including those from Cuphea, for their ability to confer jet fuel-type fatty acid accumulation in seed oil of the emerging biofuel crop Camelina sativa. Transcriptomes from Cuphea viscosissima and Cuphea pulcherrima developing seeds that accumulate >90% of C8 and C10 fatty acids revealed three FatB cDNAs (CpuFatB3, CvFatB1, and CpuFatB4) expressed predominantly in seeds and structurally divergent from typical FatB thioesterases that release 16:0 from acyl carrier protein (ACP). Expression of CpuFatB3 and CvFatB1 resulted in Camelina oil with capric acid (10:0), and CpuFatB4 expression conferred myristic acid (14:0) production and increased 16:0. Co-expression of combinations of previously characterized Cuphea and California bay FatBs produced Camelina oils with mixtures of C8-C16 fatty acids, but amounts of each fatty acid were less than obtained by expression of individual FatB cDNAs. Increases in lauric acid (12:0) and 14:0, but not 10:0, in Camelina oil and at the sn-2 position of triacylglycerols resulted from inclusion of a coconut lysophosphatidic acid acyltransferase specialized for MCFAs. RNA interference (RNAi) suppression of Camelina β-ketoacyl-ACP synthase II, however, reduced 12:0 in seeds expressing a 12:0-ACP-specific FatB. Camelina lines presented here provide platforms for additional metabolic engineering targeting fatty acid synthase and specialized acyltransferases for achieving oils with high levels of jet fuel-type fatty acids.
Keywords: Camelina; Cuphea; FatB acyl-ACP thioesterase; jet fuel oilseed; medium-chain fatty acid..
© The Author 2015. Published by Oxford University Press on behalf of the Society for Experimental Biology.
Figures


Similar articles
-
Structurally divergent lysophosphatidic acid acyltransferases with high selectivity for saturated medium chain fatty acids from Cuphea seeds.Plant J. 2015 Dec;84(5):1021-33. doi: 10.1111/tpj.13063. Plant J. 2015. PMID: 26505880
-
A Specialized Diacylglycerol Acyltransferase Contributes to the Extreme Medium-Chain Fatty Acid Content of Cuphea Seed Oil.Plant Physiol. 2017 May;174(1):97-109. doi: 10.1104/pp.16.01894. Epub 2017 Mar 21. Plant Physiol. 2017. PMID: 28325847 Free PMC article.
-
Redirection of metabolic flux for high levels of omega-7 monounsaturated fatty acid accumulation in camelina seeds.Plant Biotechnol J. 2015 Jan;13(1):38-50. doi: 10.1111/pbi.12233. Epub 2014 Jul 26. Plant Biotechnol J. 2015. PMID: 25065607
-
Cuphea: a new plant source of medium-chain fatty acids.Crit Rev Food Sci Nutr. 1989;28(2):139-73. doi: 10.1080/10408398909527495. Crit Rev Food Sci Nutr. 1989. PMID: 2653730 Review.
-
Camelina sativa: An ideal platform for the metabolic engineering and field production of industrial lipids.Biochimie. 2016 Jan;120:9-16. doi: 10.1016/j.biochi.2015.06.009. Epub 2015 Jun 21. Biochimie. 2016. PMID: 26107412 Review.
Cited by
-
Domestication and engineering of pennycress (Thlaspi arvense L.): challenges and opportunities for sustainable bio-based feedstocks.Planta. 2024 Oct 29;260(6):127. doi: 10.1007/s00425-024-04560-6. Planta. 2024. PMID: 39470818 Review.
-
Identification and Functional Characterization of Acyl-ACP Thioesterases B (GhFatBs) Responsible for Palmitic Acid Accumulation in Cotton Seeds.Int J Mol Sci. 2022 Oct 24;23(21):12805. doi: 10.3390/ijms232112805. Int J Mol Sci. 2022. PMID: 36361594 Free PMC article.
-
Stepwise metabolic engineering of Escherichia coli to produce triacylglycerol rich in medium-chain fatty acids.Biotechnol Biofuels. 2018 Jun 25;11:177. doi: 10.1186/s13068-018-1177-x. eCollection 2018. Biotechnol Biofuels. 2018. PMID: 29983740 Free PMC article.
-
Development of vegetative oil sorghum: From lab-to-field.Plant Biotechnol J. 2025 Feb;23(2):660-673. doi: 10.1111/pbi.14527. Epub 2024 Nov 30. Plant Biotechnol J. 2025. PMID: 39615039 Free PMC article.
-
Identification of Genes Involved in Lipid Biosynthesis through de novo Transcriptome Assembly from Cocos nucifera Developing Endosperm.Plant Cell Physiol. 2019 May 1;60(5):945-960. doi: 10.1093/pcp/pcy247. Plant Cell Physiol. 2019. PMID: 30608545 Free PMC article.
References
-
- Bafor M, Stymne S. 1992. Substrate specificities of glycerol acylating enzymes from developing embryos of two Cuphea species. Phytochemistry 31, 2973–2976.
-
- Bligh EG, Dyer WJ. 1959. A rapid method of total lipid extraction and purification. Canadian Journal of Biochemistry and Physiology 37, 911–917. - PubMed
-
- Cahoon EB, Dietrich CR, Meyer K, Damude HG, Dyer JM, Kinney AJ. 2006. Conjugated fatty acids accumulate to high levels in phospholipids of metabolically engineered soybean and Arabidopsis seeds, Phytochemistry 67, 1166–1176, - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

