Ganglioside biochemistry
- PMID: 25969757
- PMCID: PMC4393008
- DOI: 10.5402/2012/506160
Ganglioside biochemistry
Abstract
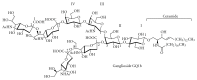
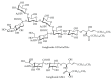
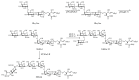
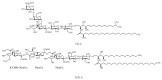
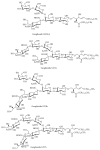
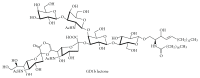
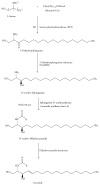
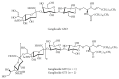
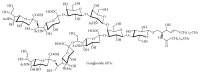
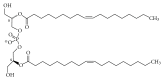
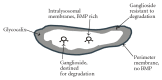
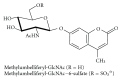
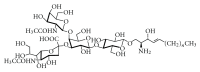
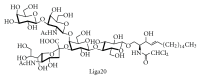
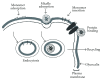
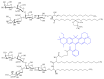
Gangliosides are sialic acid-containing glycosphingolipids. They occur especially on the cellular surfaces of neuronal cells, where they form a complex pattern, but are also found in many other cell types. The paper provides a general overview on their structures, occurrence, and metabolism. Key functional, biochemical, and pathobiochemical aspects are summarized.
Figures
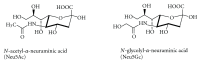
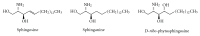



Similar articles
-
Studies on the glycosphingolipids of the starfish, Asterina pectinifera. II. Isolation and characterization of a novel ganglioside with an internal sialic acid residue.J Biochem. 1979 Aug;86(2):289-300. doi: 10.1093/oxfordjournals.jbchem.a132526. J Biochem. 1979. PMID: 113398
-
Syntheses of Fluorescent Gangliosides for the Studies of Raft Domains.Methods Enzymol. 2017;597:239-263. doi: 10.1016/bs.mie.2017.06.004. Epub 2017 Jul 18. Methods Enzymol. 2017. PMID: 28935104
-
Ganglioside structures and distribution: are they localized at the nerve ending?J Supramol Struct. 1978;8(1):1-17. doi: 10.1002/jss.400080102. J Supramol Struct. 1978. PMID: 366282 Review.
-
Expression of mouse sialic acid on gangliosides of a human glioma grown as a xenograft in SCID mice.J Neurochem. 1999 Jul;73(1):254-9. doi: 10.1046/j.1471-4159.1999.0730254.x. J Neurochem. 1999. PMID: 10386978
-
Ganglioside enhancement of neuronal differentiation, plasticity, and repair.CRC Crit Rev Clin Neurobiol. 1986;2(3):241-96. CRC Crit Rev Clin Neurobiol. 1986. PMID: 3536311 Review.
Cited by
-
Innovative and Promising Strategies to Enhance Effectiveness of Immunotherapy for CNS Tumors: Where Are We?Front Immunol. 2021 Jun 7;12:634031. doi: 10.3389/fimmu.2021.634031. eCollection 2021. Front Immunol. 2021. PMID: 34163465 Free PMC article. Review.
-
Autoantibodies in neuromuscular disorders: a review of their utility in clinical practice.Front Neurol. 2024 Nov 1;15:1495205. doi: 10.3389/fneur.2024.1495205. eCollection 2024. Front Neurol. 2024. PMID: 39555481 Free PMC article. Review.
-
Protective Effect of GM1 Attenuates Hippocampus and Cortex Apoptosis After Ketamine Exposure in Neonatal Rat via PI3K/AKT/GSK3β Pathway.Mol Neurobiol. 2021 Jul;58(7):3471-3483. doi: 10.1007/s12035-021-02346-5. Epub 2021 Mar 17. Mol Neurobiol. 2021. PMID: 33733293
-
The alteration and role of glycoconjugates in Alzheimer's disease.Front Aging Neurosci. 2024 Jun 13;16:1398641. doi: 10.3389/fnagi.2024.1398641. eCollection 2024. Front Aging Neurosci. 2024. PMID: 38946780 Free PMC article. Review.
-
Capillary Electrophoresis-Laser Induced Fluorescence Method Development and Validation for Quantification of Nine Gangliosides-Application to Analysis of Cell Lines of CNS Origin.Molecules. 2024 Aug 9;29(16):3769. doi: 10.3390/molecules29163769. Molecules. 2024. PMID: 39202849 Free PMC article.
References
-
- Miyagi T., Yamaguchi K. Sialic acids. In: Kamerling J. P., Boons G. J., Lee Y. C., Suzuki A., Taniguchi N., Voragen A. G. J., editors. Comprehensive Glycoscience. Vol. 3. Oxford, UK: Elsevier; 2007. pp. 297–322.
-
- Klenk E. Über die Ganglioside, eine neue Gruppe von zuckerhaltigen Gehirnlipoiden. Hoppe-Seyler's Zeitschrift für Physiologische Chemie. 1942;273:76–86.
-
- Klenk E. Über die Natur der Phosphatide und anderer Lipide des Gehirns und der Leber bei der Niemann-Pick'schen Krankheit. Zeitschrift für Physiologische Chemie. 1935;235:24–25.
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
