Macromolecular Crowding Enhances Catalytic Efficiency and Stability of α-Amylase
- PMID: 25969780
- PMCID: PMC4403623
- DOI: 10.5402/2013/737805
Macromolecular Crowding Enhances Catalytic Efficiency and Stability of α-Amylase
Abstract
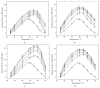
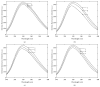
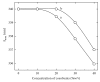
In the present study an attempt was made to investigate the macromolecular crowding effect on functional attributes of α-amylase. High concentrations of sugar based cosolvents, (e.g., trehalose, sucrose, sorbitol, and glycerol) were used to mimic the macromolecular crowding environment (of cellular milieu) under in vitro conditions. To assess the effect of macromolecular crowding, the activity and structural properties of the enzyme were evaluated in the presence of different concentrations of the above cosolvents. Based on the results it is suggested that the macromolecular crowding significantly improves the catalytic efficiency of the enzyme with marginal change in the structure. Out of four cosolvents examined, trehalose was found to be the most effective in consistently enhancing thermal stability of the enzyme. Moreover, the relative effectiveness of the above cosolvents was found to be dependent on their concentration used.
Figures


References
-
- Chang L. L., Shepherd D., Sun J., et al. Mechanism of protein stabilization by sugars during freeze-drying and storage: native structure preservation, specific interaction, and/or immobilization in a glassy matrix? Journal of Pharmaceutical Sciences. 2005;94(7):1427–1444. doi: 10.1002/jps.20364. - DOI - PubMed
-
- Ragoonanan V., Aksan A. Protein stabilization. Transfusion Medicine and Hemotherapy. 2007;34(4):246–252. doi: 10.1159/000104678. - DOI
-
- Fujita Y., Iwasa Y., Noda Y. Effect of polyhydric alcohols on invertase stabilization. Bulletin of the Chemical Society of Japan. 1984;55:1896–1900.
-
- Gekko K. Calorimetric study on thermal denaturation of lysozyme in polyol-water mixtures. Journal of Biochemistry. 1982;91(4):1197–1204. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
