Applications of nanoparticles for diagnosis and therapy of cancer
- PMID: 25969868
- PMCID: PMC4630860
- DOI: 10.1259/bjr.20150207
Applications of nanoparticles for diagnosis and therapy of cancer
Abstract
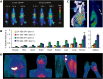
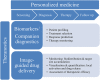
During the last decades, a plethora of nanoparticles have been developed and evaluated and a real hype has been created around their potential application as diagnostic and therapeutic agents. Despite their suggestion as potential diagnostic agents, only a single diagnostic nanoparticle formulation, namely iron oxide nanoparticles, has found its way into clinical routine so far. This fact is primarily due to difficulties in achieving appropriate pharmacokinetic properties and a reproducible synthesis of monodispersed nanoparticles. Furthermore, concerns exist about their biodegradation, elimination and toxicity. The majority of nanoparticle formulations that are currently routinely used in the clinic are used for therapeutic purposes. These therapeutic nanoparticles aim to more efficiently deliver a (chemo-) therapeutic drug to the pathological site, while avoiding its accumulation in healthy organs and tissues, and are predominantly based on the "enhanced permeability and retention" (EPR) effect. Furthermore, based on their ability to integrate diagnostic and therapeutic entities within a single nanoparticle formulation, nanoparticles hold great promise for theranostic purposes and are considered to be highly useful for personalizing nanomedicine-based treatments. In this review article, we present applications of diagnostic and therapeutic nanoparticles, summarize frequently used non-invasive imaging techniques and describe the role of EPR in the accumulation of nanotheranostic formulations. In this context, the clinical potential of nanotheranostics and image-guided drug delivery for individualized and improved (chemo-) therapeutic interventions is addressed.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

