Targeted imaging of cancer by fluorocoxib C, a near-infrared cyclooxygenase-2 probe
- PMID: 25970082
- PMCID: PMC4430360
- DOI: 10.1117/1.JBO.20.5.050502
Targeted imaging of cancer by fluorocoxib C, a near-infrared cyclooxygenase-2 probe
Abstract
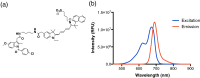
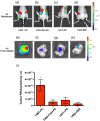
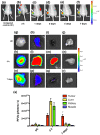
Cyclooxygenase-2 (COX-2) is a promising target for the imaging of cancer in a range of diagnostic and therapeutic settings. We report a near-infrared COX-2-targeted probe, fluorocoxib C (FC), for visualization of solid tumors by optical imaging. FC exhibits selective and potent COX-2 inhibition in both purified protein and human cancercell lines. In vivo optical imaging shows selective accumulation of FC in COX-2-overexpressing human tumor xenografts [1483 head and neck squamous cell carcinoma (HNSCC)] implanted in nude mice, while minimal uptake is detectable in COX-2-negative tumor xenografts (HCT116)or 1483 HNSCC xenografts preblocked with the COX-2-selective inhibitor celecoxib. Time course imaging studies conducted from 3 h to 7-day post-FC injection revealed a marked reduction in nonspecific fluorescent signals with retention of fluorescence in 1483 HNSCC tumors. Thus, use of FC in a delayed imaging protocol offers an approach to improve imaging signal-to-noise that should improve cancer detection in multiple preclinical and clinical settings.
Figures
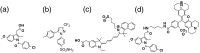
Similar articles
-
Fluorocoxib A loaded nanoparticles enable targeted visualization of cyclooxygenase-2 in inflammation and cancer.Biomaterials. 2016 Jun;92:71-80. doi: 10.1016/j.biomaterials.2016.03.028. Epub 2016 Mar 21. Biomaterials. 2016. PMID: 27043768 Free PMC article.
-
Molecular imaging of cyclooxygenase-2 in canine transitional cell carcinomas in vitro and in vivo.Cancer Prev Res (Phila). 2013 May;6(5):466-76. doi: 10.1158/1940-6207.CAPR-12-0358. Epub 2013 Mar 26. Cancer Prev Res (Phila). 2013. PMID: 23531445 Free PMC article.
-
Trifluoromethyl fluorocoxib a detects cyclooxygenase-2 expression in inflammatory tissues and human tumor xenografts.ACS Med Chem Lett. 2014 Jan 23;5(4):446-50. doi: 10.1021/ml400485g. eCollection 2014 Apr 10. ACS Med Chem Lett. 2014. PMID: 24900856 Free PMC article.
-
[Optical imaging of fluorescence in the near infrared. From passive to enzymatically activated contrast medium].Radiologe. 2007 Jan;47(1):53-61. doi: 10.1007/s00117-006-1452-x. Radiologe. 2007. PMID: 17216511 Review. German.
-
Design and construction of COX-2 specific fluorescent probes.Mol Cell Probes. 2019 Dec;48:101472. doi: 10.1016/j.mcp.2019.101472. Epub 2019 Oct 23. Mol Cell Probes. 2019. PMID: 31655104 Review.
Cited by
-
Pharmacokinetic characterization of fluorocoxib D, a cyclooxygenase-2-targeted optical imaging agent for detection of cancer.J Biomed Opt. 2020 Aug;25(8):086005. doi: 10.1117/1.JBO.25.8.086005. J Biomed Opt. 2020. PMID: 32860356 Free PMC article.
-
Polymeric Nanoparticles Enable Targeted Visualization of Drug Delivery in Breast Cancer.Mol Pharm. 2025 May 5;22(5):2392-2401. doi: 10.1021/acs.molpharmaceut.4c00695. Epub 2025 Apr 21. Mol Pharm. 2025. PMID: 40257460 Free PMC article.
-
Fluorocoxib A loaded nanoparticles enable targeted visualization of cyclooxygenase-2 in inflammation and cancer.Biomaterials. 2016 Jun;92:71-80. doi: 10.1016/j.biomaterials.2016.03.028. Epub 2016 Mar 21. Biomaterials. 2016. PMID: 27043768 Free PMC article.
-
Discovery of a Redox-Activatable Chemical Probe for Detection of Cyclooxygenase-2 in Cells and Animals.ACS Chem Biol. 2022 Jul 15;17(7):1714-1722. doi: 10.1021/acschembio.1c00961. Epub 2022 Jul 5. ACS Chem Biol. 2022. PMID: 35786843 Free PMC article.
-
Targeted Detection of Cyclooxygenase-1 in Ovarian Cancer.ACS Med Chem Lett. 2019 Jul 24;11(10):1837-1842. doi: 10.1021/acsmedchemlett.9b00280. eCollection 2020 Oct 8. ACS Med Chem Lett. 2019. PMID: 33062161 Free PMC article.
References
-
- Kubota R., et al. , “Microautoradiographic study for the differentiation of intratumoral macrophages, granulation tissues and cancer cells by the dynamics of fluorine-18-fluorodeoxyglucose uptake,” J. Nucl. Med. 35(1), 104–112 (1994).JNMEAQ - PubMed
-
- Lowe V. J., et al. , “Optimum scanning protocol for FDG-PET evaluation of pulmonary malignancy,” J. Nucl. Med. 36(5), 883–887 (1995).JNMEAQ - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

