Cytosolic extensions directly regulate a rhomboid protease by modulating substrate gating
- PMID: 25970241
- PMCID: PMC4490020
- DOI: 10.1038/nature14357
Cytosolic extensions directly regulate a rhomboid protease by modulating substrate gating
Abstract
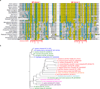
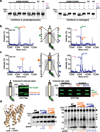
Intramembrane proteases catalyse the signal-generating step of various cell signalling pathways, and continue to be implicated in diseases ranging from malaria infection to Parkinsonian neurodegeneration. Despite playing such decisive roles, it remains unclear whether or how these membrane-immersed enzymes might be regulated directly. To address this limitation, here we focus on intramembrane proteases containing domains known to exert regulatory functions in other contexts, and characterize a rhomboid protease that harbours calcium-binding EF-hands. We find calcium potently stimulates proteolysis by endogenous rhomboid-4 in Drosophila cells, and, remarkably, when rhomboid-4 is purified and reconstituted in liposomes. Interestingly, deleting the amino-terminal EF-hands activates proteolysis prematurely, while residues in cytoplasmic loops connecting distal transmembrane segments mediate calcium stimulation. Rhomboid regulation is not orchestrated by either dimerization or substrate interactions. Instead, calcium increases catalytic rate by promoting substrate gating. Substrates with cleavage sites outside the membrane can be cleaved but lose the capacity to be regulated. These observations indicate substrate gating is not an essential step in catalysis, but instead evolved as a mechanism for regulating proteolysis inside the membrane. Moreover, these insights provide new approaches for studying rhomboid functions by investigating upstream inputs that trigger proteolysis.
Conflict of interest statement
The authors declare that no financial conflict of interest exists.
Figures






References
-
- Brown MS, Ye J, Rawson RB, Goldstein JL. Regulated intramembrane proteolysis: a control mechanism conserved from bacteria to humans. Cell. 2000;100:391–398. - PubMed
-
- De Strooper B, et al. A presenilin-1-dependent gamma-secretase-like protease mediates release of Notch intracellular domain. Nature. 1999;398:518–522. - PubMed
-
- Bier E, Jan LY, Jan Y. N. rhomboid, a gene required for dorsoventral axis establishment and peripheral nervous system development in Drosophila melanogaster. Genes Dev. 1990;4:190–203. - PubMed
-
- Lichtenthaler SF, Haass C, Steiner H. Regulated intramembrane proteolysis--lessons from amyloid precursor protein processing. J. Neurochem. 2011;117:779–796. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

