A Drosophila model identifies a critical role for zinc in mineralization for kidney stone disease
- PMID: 25970330
- PMCID: PMC4430225
- DOI: 10.1371/journal.pone.0124150
A Drosophila model identifies a critical role for zinc in mineralization for kidney stone disease
Abstract
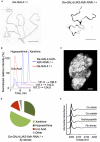
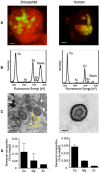
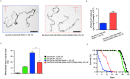
Ectopic calcification is a driving force for a variety of diseases, including kidney stones and atherosclerosis, but initiating factors remain largely unknown. Given its importance in seemingly divergent disease processes, identifying fundamental principal actors for ectopic calcification may have broad translational significance. Here we establish a Drosophila melanogaster model for ectopic calcification by inhibiting xanthine dehydrogenase whose deficiency leads to kidney stones in humans and dogs. Micro X-ray absorption near edge spectroscopy (μXANES) synchrotron analyses revealed high enrichment of zinc in the Drosophila equivalent of kidney stones, which was also observed in human kidney stones and Randall's plaques (early calcifications seen in human kidneys thought to be the precursor for renal stones). To further test the role of zinc in driving mineralization, we inhibited zinc transporter genes in the ZnT family and observed suppression of Drosophila stone formation. Taken together, genetic, dietary, and pharmacologic interventions to lower zinc confirm a critical role for zinc in driving the process of heterogeneous nucleation that eventually leads to stone formation. Our findings open a novel perspective on the etiology of urinary stones and related diseases, which may lead to the identification of new preventive and therapeutic approaches.
Conflict of interest statement
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

