Antibiotics in early life alter the gut microbiome and increase disease incidence in a spontaneous mouse model of autoimmune insulin-dependent diabetes
- PMID: 25970503
- PMCID: PMC4430542
- DOI: 10.1371/journal.pone.0125448
Antibiotics in early life alter the gut microbiome and increase disease incidence in a spontaneous mouse model of autoimmune insulin-dependent diabetes
Erratum in
-
Correction: Antibiotics in Early Life Alter the Gut Microbiome and Increase Disease Incidence in a Spontaneous Mouse Model of Autoimmune Insulin-Dependent Diabetes.PLoS One. 2016 Jan 22;11(1):e0147888. doi: 10.1371/journal.pone.0147888. eCollection 2016. PLoS One. 2016. PMID: 26799316 Free PMC article. No abstract available.
Abstract
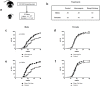
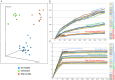
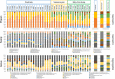
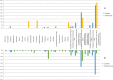
Insulin-dependent or type 1 diabetes is a prototypic autoimmune disease whose incidence steadily increased over the past decades in industrialized countries. Recent evidence suggests the importance of the gut microbiota to explain this trend. Here, non-obese diabetic (NOD) mice that spontaneously develop autoimmune type 1 diabetes were treated with different antibiotics to explore the influence of a targeted intestinal dysbiosis in the progression of the disease. A mixture of wide spectrum antibiotics (i.e. streptomycin, colistin and ampicillin) or vancomycin alone were administered orally from the moment of conception, treating breeding pairs, and during the postnatal and adult life until the end of follow-up at 40 weeks. Diabetes incidence significantly and similarly increased in male mice following treatment with these two antibiotic regimens. In NOD females a slight yet not significant trend towards an increase in disease incidence was observed. Changes in gut microbiota composition were assessed by sequencing the V3 region of bacterial 16S rRNA genes. Administration of the antibiotic mixture resulted in near complete ablation of the gut microbiota. Vancomycin treatment led to increased Escherichia, Lactobacillus and Sutterella genera and decreased members of the Clostridiales order and Lachnospiraceae, Prevotellaceae and Rikenellaceae families, as compared to control mice. Massive elimination of IL-17-producing cells, both CD4+TCRαβ+ and TCRγδ+ T cells was observed in the lamina propria of the ileum and the colon of vancomycin-treated mice. These results show that a directed even partial ablation of the gut microbiota, as induced by vancomycin, significantly increases type 1 diabetes incidence in male NOD mice thus prompting for caution in the use of antibiotics in pregnant women and newborns.
Conflict of interest statement
Figures

Similar articles
-
Prolonged antibiotic treatment induces a diabetogenic intestinal microbiome that accelerates diabetes in NOD mice.ISME J. 2016 Feb;10(2):321-32. doi: 10.1038/ismej.2015.114. Epub 2015 Aug 14. ISME J. 2016. PMID: 26274050 Free PMC article.
-
Different immunological responses to early-life antibiotic exposure affecting autoimmune diabetes development in NOD mice.J Autoimmun. 2016 Aug;72:47-56. doi: 10.1016/j.jaut.2016.05.001. Epub 2016 May 10. J Autoimmun. 2016. PMID: 27178773 Free PMC article.
-
Antibiotic-associated dysbiosis affects the ability of the gut microbiota to control intestinal inflammation upon fecal microbiota transplantation in experimental colitis models.Microbiome. 2021 Feb 6;9(1):39. doi: 10.1186/s40168-020-00991-x. Microbiome. 2021. PMID: 33549144 Free PMC article.
-
Targeting gut microbiota as a possible therapy for diabetes.Nutr Res. 2015 May;35(5):361-7. doi: 10.1016/j.nutres.2015.03.002. Epub 2015 Mar 14. Nutr Res. 2015. PMID: 25818484 Review.
-
Effect of antibiotics on gut microbiota, glucose metabolism and body weight regulation: a review of the literature.Diabetes Obes Metab. 2016 May;18(5):444-53. doi: 10.1111/dom.12637. Epub 2016 Mar 4. Diabetes Obes Metab. 2016. PMID: 26818734 Review.
Cited by
-
The gut microbiome of laboratory mice: considerations and best practices for translational research.Mamm Genome. 2021 Aug;32(4):239-250. doi: 10.1007/s00335-021-09863-7. Epub 2021 Mar 10. Mamm Genome. 2021. PMID: 33689000 Free PMC article. Review.
-
Emerging Technologies for Gut Microbiome Research.Trends Microbiol. 2016 Nov;24(11):887-901. doi: 10.1016/j.tim.2016.06.008. Epub 2016 Jul 15. Trends Microbiol. 2016. PMID: 27426971 Free PMC article. Review.
-
Metagenome-Assembled Genomes from Murine Fecal Microbiomes Dominated by Uncharacterized Bacteria.Microbiol Resour Announc. 2023 Mar 16;12(3):e0116222. doi: 10.1128/mra.01162-22. Epub 2023 Feb 13. Microbiol Resour Announc. 2023. PMID: 36779794 Free PMC article.
-
Gut microbiome in type 1 diabetes: A comprehensive review.Diabetes Metab Res Rev. 2018 Oct;34(7):e3043. doi: 10.1002/dmrr.3043. Epub 2018 Jul 17. Diabetes Metab Res Rev. 2018. PMID: 29929213 Free PMC article. Review.
-
The antibiotic vancomycin induces complexation and aggregation of gastrointestinal and submaxillary mucins.Sci Rep. 2020 Jan 22;10(1):960. doi: 10.1038/s41598-020-57776-3. Sci Rep. 2020. PMID: 31969624 Free PMC article.
References
-
- Bach JF The effect of infections on susceptibility to autoimmune and allergic diseases. N Engl J Med. 2002; 347: 911–920. - PubMed
-
- Nylund L, Satokari R, Nikkila J, Rajilic-Stojanovic M, Kalliomaki M, Isolauri E, et al. Microarray analysis reveals marked intestinal microbiota aberrancy in infants having eczema compared to healthy children in at-risk for atopic disease. BMC Microbiol. 2013; 13: 12 10.1186/1471-2180-13-12 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

