Development and validation of a biomarker for diarrhea-predominant irritable bowel syndrome in human subjects
- PMID: 25970536
- PMCID: PMC4430499
- DOI: 10.1371/journal.pone.0126438
Development and validation of a biomarker for diarrhea-predominant irritable bowel syndrome in human subjects
Abstract
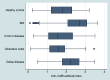
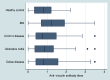
Diarrhea-predominant irritable bowel syndrome (IBS) is diagnosed through clinical criteria after excluding "organic" conditions, and can be precipitated by acute gastroenteritis. Cytolethal distending toxin B (CdtB) is produced by bacteria that cause acute gastroenteritis, and a post-infectious animal model demonstrates that host antibodies to CdtB cross-react with vinculin in the host gut, producing an IBS-like phenotype. Therefore, we assessed circulating anti-CdtB and anti-vinculin antibodies as biomarkers for D-IBS in human subjects. Subjects with D-IBS based on Rome criteria (n=2375) were recruited from a large-scale multicenter clinical trial for D-IBS (TARGET 3). Subjects with inflammatory bowel disease (IBD) (n=142), subjects with celiac disease (n=121), and healthy controls (n=43) were obtained for comparison. Subjects with IBD and celiac disease were recruited based on the presence of intestinal complaints and histologic confirmation of chronic inflammatory changes in the colon or small intestine. Subjects with celiac disease were also required to have an elevated tTG and biopsy. All subjects were aged between 18 and 65 years. Plasma levels of anti-CdtB and anti-vinculin antibodies were determined by ELISA, and compared between groups. Anti-CdtB titers were significantly higher in D-IBS subjects compared to IBD, healthy controls and celiac disease (P<0.001). Anti-vinculin titers were also significantly higher in IBS (P<0.001) compared to the other groups. The area-under-the-receiver operating curves (AUCs) were 0.81 and 0.62 for diagnosis of D-IBS against IBD for anti-CdtB and anti-vinculin, respectively. Both tests were less specific in differentiating IBS from celiac disease. Optimization demonstrated that for anti-CdtB (optical density≥2.80) the specificity, sensitivity and likelihood ratio were 91.6%, 43.7 and 5.2, respectively, and for anti-vinculin (OD≥1.68) were 83.8%, 32.6 and 2.0, respectively. These results confirm that anti-CdtB and anti-vinculin antibodies are elevated in D-IBS compared to non-IBS subjects. These biomarkers may be especially helpful in distinguishing D-IBS from IBD in the workup of chronic diarrhea.
Conflict of interest statement
Figures

Comment in
-
Validation of a Serum Biomarker for Irritable Bowel Syndrome: Is an Accurate, Single Biomarker Test Possible?Gastroenterology. 2016 Jan;150(1):277-8. doi: 10.1053/j.gastro.2015.10.010. Epub 2015 Oct 20. Gastroenterology. 2016. PMID: 26496467 No abstract available.
-
[A diagnostic blood test for irritable bowel syndrome: a utopia?].Rev Med Suisse. 2015 Sep 2;11(484):1628. Rev Med Suisse. 2015. PMID: 26502628 French. No abstract available.
-
Reply.Gastroenterology. 2016 Jan;150(1):278-9. doi: 10.1053/j.gastro.2015.11.023. Epub 2015 Nov 21. Gastroenterology. 2016. PMID: 26613888 No abstract available.
References
-
- Hill ID. What are the sensitivity and specificity of serologic tests for celiac disease? Do sensitivity and specificity vary in different populations? Gastroenterology 2005;128: S25–32. - PubMed
-
- Kruis W, Thieme C, Weinzierl M, Schussler P, Holl J, Paulus W. A diagnostic score for the irritable bowel syndrome. Its value in the exclusion of organic disease. Gastroenterology 1984;87: 1–7. - PubMed
-
- Thompson WG, Dotevall G, Drossman DA, Heaton KW, Kruis W. Irritable bowel syndrome: Guidelines for diagnosis. Gastroenterol Int 1989;2: 92–95.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

