Oxidative stress and the homeodynamics of iron metabolism
- PMID: 25970586
- PMCID: PMC4496698
- DOI: 10.3390/biom5020808
Oxidative stress and the homeodynamics of iron metabolism
Abstract
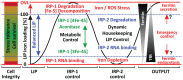
Iron and oxygen share a delicate partnership since both are indispensable for survival, but if the partnership becomes inadequate, this may rapidly terminate life. Virtually all cell components are directly or indirectly affected by cellular iron metabolism, which represents a complex, redox-based machinery that is controlled by, and essential to, metabolic requirements. Under conditions of increased oxidative stress—i.e., enhanced formation of reactive oxygen species (ROS)—however, this machinery may turn into a potential threat, the continued requirement for iron promoting adverse reactions such as the iron/H2O2-based formation of hydroxyl radicals, which exacerbate the initial pro-oxidant condition. This review will discuss the multifaceted homeodynamics of cellular iron management under normal conditions as well as in the context of oxidative stress.
Keywords: iron; metabolism; oxidative stress.
Figures

References
-
- Conrad M.E., Umbreit J.N., Moore E.G., Hainsworth L.N., Porubcin M., Simovich M.J., Nakada M.T., Dolan K., Garrick M.D. Separate pathways for cellular uptake of ferric and ferrous iron. Am. J. Physiol. Gastrointest. Liver Physiol. 2000;279:G767–G774. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

