Hcn1 is a tremorgenic genetic component in a rat model of essential tremor
- PMID: 25970616
- PMCID: PMC4430019
- DOI: 10.1371/journal.pone.0123529
Hcn1 is a tremorgenic genetic component in a rat model of essential tremor
Expression of concern in
-
Expression of Concern: Hcn1 Is a Tremorgenic Genetic Component in a Rat Model of Essential Tremor.PLoS One. 2023 Jan 11;18(1):e0279459. doi: 10.1371/journal.pone.0279459. eCollection 2023. PLoS One. 2023. PMID: 36630358 Free PMC article. No abstract available.
Abstract
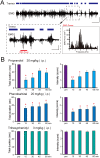
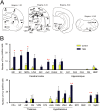
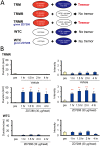
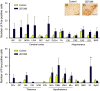
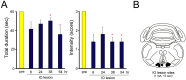
Genetic factors are thought to play a major role in the etiology of essential tremor (ET); however, few genetic changes that induce ET have been identified to date. In the present study, to find genes responsible for the development of ET, we employed a rat model system consisting of a tremulous mutant strain, TRM/Kyo (TRM), and its substrain TRMR/Kyo (TRMR). The TRM rat is homozygous for the tremor (tm) mutation and shows spontaneous tremors resembling human ET. The TRMR rat also carries a homozygous tm mutation but shows no tremor, leading us to hypothesize that TRM rats carry one or more genes implicated in the development of ET in addition to the tm mutation. We used a positional cloning approach and found a missense mutation (c. 1061 C>T, p. A354V) in the hyperpolarization-activated cyclic nucleotide-gated 1 channel (Hcn1) gene. The A354V HCN1 failed to conduct hyperpolarization-activated currents in vitro, implicating it as a loss-of-function mutation. Blocking HCN1 channels with ZD7288 in vivo evoked kinetic tremors in nontremulous TRMR rats. We also found neuronal activation of the inferior olive (IO) in both ZD7288-treated TRMR and non-treated TRM rats and a reduced incidence of tremor in the IO-lesioned TRM rats, suggesting a critical role of the IO in tremorgenesis. A rat strain carrying the A354V mutation alone on a genetic background identical to that of the TRM rats showed no tremor. Together, these data indicate that body tremors emerge when the two mutant loci, tm and Hcn1A354V, are combined in a rat model of ET. In this model, HCN1 channels play an important role in the tremorgenesis of ET. We propose that oligogenic, most probably digenic, inheritance is responsible for the genetic heterogeneity of ET.
Conflict of interest statement
Figures

Similar articles
-
Involvement of aspartoacylase in tremor expression in rats.Exp Anim. 2016 Jul 29;65(3):293-301. doi: 10.1538/expanim.16-0007. Epub 2016 Mar 30. Exp Anim. 2016. PMID: 27026062 Free PMC article.
-
Involvement of NMDA receptors in tremor expression in Aspa/Hcn1 double-knockout rats.Exp Anim. 2020 Nov 12;69(4):388-394. doi: 10.1538/expanim.20-0025. Epub 2020 Jun 5. Exp Anim. 2020. PMID: 32507787 Free PMC article.
-
Loss of HCN1 subunits causes absence epilepsy in rats.Brain Res. 2019 Mar 1;1706:209-217. doi: 10.1016/j.brainres.2018.11.004. Epub 2018 Nov 5. Brain Res. 2019. PMID: 30408474
-
The Impact of Altered HCN1 Expression on Brain Function and Its Relationship with Epileptogenesis.Curr Neuropharmacol. 2023;21(10):2070-2078. doi: 10.2174/1570159X21666230214110333. Curr Neuropharmacol. 2023. PMID: 37366350 Free PMC article. Review.
-
Molecular regulation and pharmacology of pacemaker channels.Curr Pharm Des. 2007;13(23):2338-49. doi: 10.2174/138161207781368729. Curr Pharm Des. 2007. PMID: 17692005 Review.
Cited by
-
Rat models of human diseases and related phenotypes: a systematic inventory of the causative genes.J Biomed Sci. 2020 Aug 2;27(1):84. doi: 10.1186/s12929-020-00673-8. J Biomed Sci. 2020. PMID: 32741357 Free PMC article. Review.
-
Mechanism Underlying Organophosphate Paraoxon-Induced Kinetic Tremor.Neurotox Res. 2019 Apr;35(3):575-583. doi: 10.1007/s12640-019-0007-7. Epub 2019 Feb 7. Neurotox Res. 2019. PMID: 30729450
-
Involvement of aspartoacylase in tremor expression in rats.Exp Anim. 2016 Jul 29;65(3):293-301. doi: 10.1538/expanim.16-0007. Epub 2016 Mar 30. Exp Anim. 2016. PMID: 27026062 Free PMC article.
-
Animal Models of Tremor: Relevance to Human Tremor Disorders.Tremor Other Hyperkinet Mov (N Y). 2018 Oct 9;8:587. doi: 10.7916/D89S37MV. eCollection 2018. Tremor Other Hyperkinet Mov (N Y). 2018. PMID: 30402338 Free PMC article. Review.
-
Nicotine Elicits Convulsive Seizures by Activating Amygdalar Neurons.Front Pharmacol. 2017 Feb 9;8:57. doi: 10.3389/fphar.2017.00057. eCollection 2017. Front Pharmacol. 2017. PMID: 28232801 Free PMC article.
References
-
- Benito-Leon J, Louis ED (2006) Essential tremor: emerging views of a common disorder. Nat Clin Pract Neurol 2: 666–678. - PubMed
-
- Louis ED (2001) Etiology of essential tremor: should we be searching for environmental causes? Mov Disord 16: 822–829. - PubMed
-
- Jimenez-Jimenez FJ, Alonso-Navarro H, Garcia-Martin E, Lorenzo-Betancor O, Pastor P, et al. (2013) Update on genetics of essential tremor. Acta Neurol Scand. - PubMed
-
- Gulcher JR, Jonsson P, Kong A, Kristjansson K, Frigge ML, et al. (1997) Mapping of a familial essential tremor gene, FET1, to chromosome 3q13. Nat Genet 17: 84–87. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

