Breaking a pathogen's iron will: Inhibiting siderophore production as an antimicrobial strategy
- PMID: 25970810
- PMCID: PMC4457648
- DOI: 10.1016/j.bbapap.2015.05.001
Breaking a pathogen's iron will: Inhibiting siderophore production as an antimicrobial strategy
Abstract
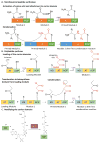
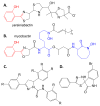
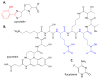
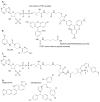
The rise of antibiotic resistance is a growing public health crisis. Novel antimicrobials are sought, preferably developing nontraditional chemical scaffolds that do not inhibit standard targets such as cell wall synthesis or the ribosome. Iron scavenging has been proposed as a viable target, because bacterial and fungal pathogens must overcome the nutritional immunity of the host to be virulent. This review highlights the recent work toward exploiting the biosynthetic enzymes of siderophore production for the design of next generation antimicrobials.
Keywords: NRPS-independent synthetase; Nonribosomal peptide synthetase; Polyketide synthase; Siderophore.
Copyright © 2015 Elsevier B.V. All rights reserved.
Figures
Similar articles
-
Transferrin-mediated iron sequestration as a novel therapy for bacterial and fungal infections.Curr Opin Microbiol. 2015 Oct;27:57-61. doi: 10.1016/j.mib.2015.07.005. Epub 2015 Aug 9. Curr Opin Microbiol. 2015. PMID: 26261881 Free PMC article. Review.
-
Targeting Siderophore Biosynthesis to Thwart Microbial Growth.Int J Mol Sci. 2025 Apr 11;26(8):3611. doi: 10.3390/ijms26083611. Int J Mol Sci. 2025. PMID: 40332123 Free PMC article. Review.
-
Building a Trojan Horse: Siderophore-Drug Conjugates for the Treatment of Infectious Diseases.Met Ions Life Sci. 2019 Jan 14;19:/books/9783110527872/9783110527872-013/9783110527872-013.xml. doi: 10.1515/9783110527872-013. Met Ions Life Sci. 2019. PMID: 30855108 Review.
-
[Antimicrobial resistance].Internist (Berl). 2015 Nov;56(11):1231-2. doi: 10.1007/s00108-015-3709-9. Internist (Berl). 2015. PMID: 26507817 German. No abstract available.
-
Siderophores; iron scavengers: the novel & promising targets for pathogen specific antifungal therapy.Expert Opin Ther Targets. 2016 Dec;20(12):1477-1489. doi: 10.1080/14728222.2016.1254196. Epub 2016 Nov 11. Expert Opin Ther Targets. 2016. PMID: 27797604 Review.
Cited by
-
Amino Acid Biosynthetic Pathways Are Required for Klebsiella pneumoniae Growth in Immunocompromised Lungs and Are Druggable Targets during Infection.Antimicrob Agents Chemother. 2019 Jul 25;63(8):e02674-18. doi: 10.1128/AAC.02674-18. Print 2019 Aug. Antimicrob Agents Chemother. 2019. PMID: 31109974 Free PMC article.
-
Antifungal Drugs.Metabolites. 2020 Mar 12;10(3):106. doi: 10.3390/metabo10030106. Metabolites. 2020. PMID: 32178468 Free PMC article. Review.
-
Siderophore conjugates to combat antibiotic-resistant bacteria.RSC Med Chem. 2023 Mar 1;14(5):800-822. doi: 10.1039/d2md00465h. eCollection 2023 May 25. RSC Med Chem. 2023. PMID: 37252105 Free PMC article. Review.
-
Kinetic analysis of the three-substrate reaction mechanism of an NRPS-independent siderophore (NIS) synthetase.Methods Enzymol. 2024;702:1-19. doi: 10.1016/bs.mie.2024.06.012. Epub 2024 Jul 3. Methods Enzymol. 2024. PMID: 39155107 Free PMC article.
-
Connecting iron acquisition and biofilm formation in the ESKAPE pathogens as a strategy for combatting antibiotic resistance.Medchemcomm. 2019 Mar 21;10(4):505-512. doi: 10.1039/c9md00032a. eCollection 2019 Apr 1. Medchemcomm. 2019. PMID: 31057729 Free PMC article. Review.
References
-
- The White House Executive Order -- Combating Antibiotic-Resistant Bacteria. https://www.whitehouse.gov/the-press-office/2014/09/18/executive-order-c....
-
- Centers for Disease Control and Prevention Antibiotic Resistance Solutions Initiative. http://www.cdc.gov/drugresistance/solutions-initiative/index.html.
-
- World Health Organization. Antimicrobial Resistance: Global Report on Surveillance. 2014.
-
- Clatworthy AE, Pierson E, Hung DT. Targeting virulence: a new paradigm for antimicrobial therapy. Nat Chem Biol. 2007;3(9):541–8. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

