Marginally hydrophobic transmembrane α-helices shaping membrane protein folding
- PMID: 25970811
- PMCID: PMC4500307
- DOI: 10.1002/pro.2698
Marginally hydrophobic transmembrane α-helices shaping membrane protein folding
Abstract
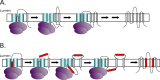
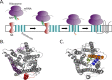
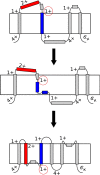
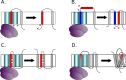
Cells have developed an incredible machinery to facilitate the insertion of membrane proteins into the membrane. While we have a fairly good understanding of the mechanism and determinants of membrane integration, more data is needed to understand the insertion of membrane proteins with more complex insertion and folding pathways. This review will focus on marginally hydrophobic transmembrane helices and their influence on membrane protein folding. These weakly hydrophobic transmembrane segments are by themselves not recognized by the translocon and therefore rely on local sequence context for membrane integration. How can such segments reside within the membrane? We will discuss this in the light of features found in the protein itself as well as the environment it resides in. Several characteristics in proteins have been described to influence the insertion of marginally hydrophobic helices. Additionally, the influence of biological membranes is significant. To begin with, the actual cost for having polar groups within the membrane may not be as high as expected; the presence of proteins in the membrane as well as characteristics of some amino acids may enable a transmembrane helix to harbor a charged residue. The lipid environment has also been shown to directly influence the topology as well as membrane boundaries of transmembrane helices-implying a dynamic relationship between membrane proteins and their environment.
Keywords: aquaporin 1; hydrophobicity; marginally hydrophobic transmembrane helices; membrane protein folding.
© 2015 The Protein Society.
Figures

Similar articles
-
Membrane insertion of marginally hydrophobic transmembrane helices depends on sequence context.J Mol Biol. 2010 Feb 12;396(1):221-9. doi: 10.1016/j.jmb.2009.11.036. Epub 2009 Nov 18. J Mol Biol. 2010. PMID: 19931281
-
The positive inside rule is stronger when followed by a transmembrane helix.J Mol Biol. 2014 Aug 12;426(16):2982-91. doi: 10.1016/j.jmb.2014.06.002. Epub 2014 Jun 10. J Mol Biol. 2014. PMID: 24927974
-
Recognition of transmembrane helices by the endoplasmic reticulum translocon.Nature. 2005 Jan 27;433(7024):377-81. doi: 10.1038/nature03216. Nature. 2005. PMID: 15674282
-
Amphipathic helices and membrane curvature.FEBS Lett. 2010 May 3;584(9):1840-7. doi: 10.1016/j.febslet.2009.10.022. Epub 2009 Oct 20. FEBS Lett. 2010. PMID: 19837069 Review.
-
Folding and Insertion of Transmembrane Helices at the ER.Int J Mol Sci. 2021 Nov 26;22(23):12778. doi: 10.3390/ijms222312778. Int J Mol Sci. 2021. PMID: 34884581 Free PMC article. Review.
Cited by
-
Discovery and Characterization of the Phospholemman/SIMP/Viroporin Superfamily.Microb Physiol. 2022;32(3-4):83-94. doi: 10.1159/000521947. Epub 2022 Feb 11. Microb Physiol. 2022. PMID: 35152214 Free PMC article.
-
Refined topology model of the STT3/Stt3 protein subunit of the oligosaccharyltransferase complex.J Biol Chem. 2017 Jul 7;292(27):11349-11360. doi: 10.1074/jbc.M117.779421. Epub 2017 May 16. J Biol Chem. 2017. PMID: 28512128 Free PMC article.
-
The Landscape of Pseudomonas aeruginosa Membrane-Associated Proteins.Cells. 2020 Nov 5;9(11):2421. doi: 10.3390/cells9112421. Cells. 2020. PMID: 33167383 Free PMC article.
-
Interaction mapping of the Sec61 translocon identifies two Sec61α regions interacting with hydrophobic segments in translocating chains.J Biol Chem. 2018 Nov 2;293(44):17050-17060. doi: 10.1074/jbc.RA118.003219. Epub 2018 Sep 13. J Biol Chem. 2018. PMID: 30213864 Free PMC article.
-
Relax, Cool Down and Scaffold: How to Restore Surface Expression of Folding-Deficient Mutant GPCRs and SLC6 Transporters.Int J Mol Sci. 2017 Nov 14;18(11):2416. doi: 10.3390/ijms18112416. Int J Mol Sci. 2017. PMID: 29135937 Free PMC article. Review.
References
-
- Seppala S, Slusky JS, Lloris-Garcera P, Rapp M, von Heijne G. Control of membrane protein topology by a single C-terminal residue. Science. 2010;328:1698–1700. - PubMed
-
- Pauling L. The nature of the chemical bond, Addison-Wesley Edition. Vol. 2. New York: Cornell University Press; 1939.
-
- Wernet P, Nordlund D, Bergmann U, Cavalleri M, Odelius M, Ogasawara H, Näslund LR, Hirsch TK, Ojamäe L, Glatzel P, Pettersson LGM, Nilsson A. The structure of the first coordination shell in liquid water. Science. 2004;304:995–999. - PubMed
-
- Israelachvili JN, editor. Intermolecular and surface forces. 3rd ed. Boston: Academic Press; 2011. , editor (
-
- Lide DR. CRC handbook of chemistry and physics. 88th ed. CRC Press; 2007.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

