Marginally hydrophobic transmembrane α-helices shaping membrane protein folding
- PMID: 25970811
- PMCID: PMC4500307
- DOI: 10.1002/pro.2698
Marginally hydrophobic transmembrane α-helices shaping membrane protein folding
Abstract
Cells have developed an incredible machinery to facilitate the insertion of membrane proteins into the membrane. While we have a fairly good understanding of the mechanism and determinants of membrane integration, more data is needed to understand the insertion of membrane proteins with more complex insertion and folding pathways. This review will focus on marginally hydrophobic transmembrane helices and their influence on membrane protein folding. These weakly hydrophobic transmembrane segments are by themselves not recognized by the translocon and therefore rely on local sequence context for membrane integration. How can such segments reside within the membrane? We will discuss this in the light of features found in the protein itself as well as the environment it resides in. Several characteristics in proteins have been described to influence the insertion of marginally hydrophobic helices. Additionally, the influence of biological membranes is significant. To begin with, the actual cost for having polar groups within the membrane may not be as high as expected; the presence of proteins in the membrane as well as characteristics of some amino acids may enable a transmembrane helix to harbor a charged residue. The lipid environment has also been shown to directly influence the topology as well as membrane boundaries of transmembrane helices-implying a dynamic relationship between membrane proteins and their environment.
Keywords: aquaporin 1; hydrophobicity; marginally hydrophobic transmembrane helices; membrane protein folding.
© 2015 The Protein Society.
Figures
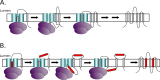

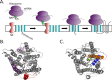
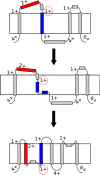
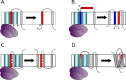
References
-
- Seppala S, Slusky JS, Lloris-Garcera P, Rapp M, von Heijne G. Control of membrane protein topology by a single C-terminal residue. Science. 2010;328:1698–1700. - PubMed
-
- Pauling L. The nature of the chemical bond, Addison-Wesley Edition. Vol. 2. New York: Cornell University Press; 1939.
-
- Wernet P, Nordlund D, Bergmann U, Cavalleri M, Odelius M, Ogasawara H, Näslund LR, Hirsch TK, Ojamäe L, Glatzel P, Pettersson LGM, Nilsson A. The structure of the first coordination shell in liquid water. Science. 2004;304:995–999. - PubMed
-
- Israelachvili JN, editor. Intermolecular and surface forces. 3rd ed. Boston: Academic Press; 2011. , editor (
-
- Lide DR. CRC handbook of chemistry and physics. 88th ed. CRC Press; 2007.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

