Type II transglutaminase stimulates epidermal cancer stem cell epithelial-mesenchymal transition
- PMID: 25971211
- PMCID: PMC4653023
- DOI: 10.18632/oncotarget.3890
Type II transglutaminase stimulates epidermal cancer stem cell epithelial-mesenchymal transition
Abstract
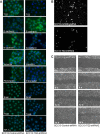
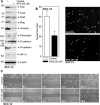
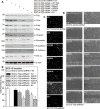
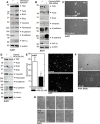
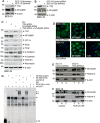
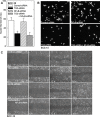
Type II transglutaminase (TG2) is a multifunctional protein that has recently been implicated as having a role in ECS cell survival. In the present study we investigate the role of TG2 in regulating epithelial mesenchymal transition (EMT) in ECS cells. Our studies show that TG2 knockdown or treatment with TG2 inhibitor, results in a reduced EMT marker expression, and reduced cell migration and invasion. TG2 has several activities, but the most prominent are its transamidase and GTP binding activity. Analysis of a series of TG2 mutants reveals that TG2 GTP binding activity, but not the transamidase activity, is required for expression of EMT markers (Twist, Snail, Slug, vimentin, fibronectin, N-cadherin and HIF-1α), and increased ECS cell invasion and migration. This coupled with reduced expression of E-cadherin. Additional studies indicate that NFÏ°B signaling, which has been implicated as mediating TG2 impact on EMT in breast cancer cells, is not involved in TG2 regulation of EMT in skin cancer. These studies suggest that TG2 is required for maintenance of ECS cell EMT, invasion and migration, and suggests that inhibiting TG2 GTP binding/G-protein related activity may reduce skin cancer tumor survival.
Keywords: TG2; cancer stem cell; epidermal squamous cell carcinoma; epithelial mesenchymal transition; type II transglutaminase.
Figures

References
-
- Christenson LJ, Borrowman TA, Vachon CM, Tollefson MM, Otley CC, Weaver AL, Roenigk RK. Incidence of basal cell and squamous cell carcinomas in a population younger than 40 years. JAMA. 2005;294:681–690. - PubMed
-
- Moller R, Reymann F, Hou-Jensen K. Metastases in dermatological patients with squamous cell carcinoma. Arch Dermatol. 1979;115:703–705. - PubMed
-
- Chaffer CL, Thompson EW, Williams ED. Mesenchymal to epithelial transition in development and disease. Cells Tissues Organs. 2007;185:7–19. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

