Global Shifts in Genome and Proteome Composition Are Very Tightly Coupled
- PMID: 25971281
- PMCID: PMC4494046
- DOI: 10.1093/gbe/evv088
Global Shifts in Genome and Proteome Composition Are Very Tightly Coupled
Abstract
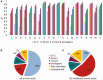
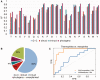
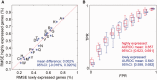
The amino acid composition (AAC) of proteomes differs greatly between microorganisms and is associated with the environmental niche they inhabit, suggesting that these changes may be adaptive. Similarly, the oligonucleotide composition of genomes varies and may confer advantages at the DNA/RNA level. These influences overlap in protein-coding sequences, making it difficult to gauge their relative contributions. We disentangle these effects by systematically evaluating the correspondence between intergenic nucleotide composition, where protein-level selection is absent, the AAC, and ecological parameters of 909 prokaryotes. We find that G + C content, the most frequently used measure of genomic composition, cannot capture diversity in AAC and across ecological contexts. However, di-/trinucleotide composition in intergenic DNA predicts amino acid frequencies of proteomes to the point where very little cross-species variability remains unexplained (91% of variance accounted for). Qualitatively similar results were obtained for 49 fungal genomes, where 80% of the variability in AAC could be explained by the composition of introns and intergenic regions. Upon factoring out oligonucleotide composition and phylogenetic inertia, the residual AAC is poorly predictive of the microbes' ecological preferences, in stark contrast with the original AAC. Moreover, highly expressed genes do not exhibit more prominent environment-related AAC signatures than lowly expressed genes, despite contributing more to the effective proteome. Thus, evolutionary shifts in overall AAC appear to occur almost exclusively through factors shaping the global oligonucleotide content of the genome. We discuss these results in light of contravening evidence from biophysical data and further reading frame-specific analyses that suggest that adaptation takes place at the protein level.
Keywords: amino acid composition; ecological preferences; fungal genome; intergenic DNA; oligonucleotide composition; prokaryotic genome; support vector regression.
© The Author(s) 2015. Published by Oxford University Press on behalf of the Society for Molecular Biology and Evolution.
Figures




References
-
- Berka RM, et al. 2011. Comparative genomic analysis of the thermophilic biomass-degrading fungi Myceliophthora thermophila and Thielavia terrestris. Nat Biotech. 29:922–927. - PubMed
-
- Blockeel H, Raedt LD, Ramon J. 1998. Top-down induction of clustering trees. In: Shavlik JW, editor. Proceedings of the Fifteenth International Conference on Machine Learning. ICML ’98; Madison, Wisconsin. San Francisco (CA): Morgan Kaufmann Publishers Inc. p. 55–63. Available from: http://dl.acm.org/citation.cfm?id=645527.657456.
-
- Breiman L. 2001. Random forests. Mach Learn. 45:5–32.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

