Pharmacological targeting of miR-155 via the NEDD8-activating enzyme inhibitor MLN4924 (Pevonedistat) in FLT3-ITD acute myeloid leukemia
- PMID: 25971362
- PMCID: PMC4868182
- DOI: 10.1038/leu.2015.106
Pharmacological targeting of miR-155 via the NEDD8-activating enzyme inhibitor MLN4924 (Pevonedistat) in FLT3-ITD acute myeloid leukemia
Abstract
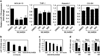
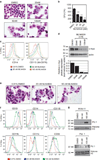
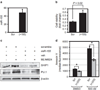
High levels of microRNA-155 (miR-155) are associated with poor outcome in acute myeloid leukemia (AML). In AML, miR-155 is regulated by NF-κB, the activity of which is, in part, controlled by the NEDD8-dependent ubiquitin ligases. We demonstrate that MLN4924, an inhibitor of NEDD8-activating enzyme presently being evaluated in clinical trials, decreases binding of NF-κB to the miR-155 promoter and downregulates miR-155 in AML cells. This results in the upregulation of the miR-155 targets SHIP1, an inhibitor of the PI3K/Akt pathway, and PU.1, a transcription factor important for myeloid differentiation, leading to monocytic differentiation and apoptosis. Consistent with these results, overexpression of miR-155 diminishes MLN4924-induced antileukemic effects. In vivo, MLN4924 reduces miR-155 expression and prolongs the survival of mice engrafted with leukemic cells. Our study demonstrates the potential of miR-155 as a novel therapeutic target in AML via pharmacologic interference with NF-κB-dependent regulatory mechanisms. We show the targeting of this oncogenic microRNA with MLN4924, a compound presently being evaluated in clinical trials in AML. As high miR-155 levels have been consistently associated with aggressive clinical phenotypes, our work opens new avenues for microRNA-targeting therapeutic approaches to leukemia and cancer patients.
Conflict of interest statement
CONFLICT OF INTEREST
The authors declare no conflict of interest.
Figures
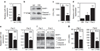




Similar articles
-
Silvestrol exhibits significant in vivo and in vitro antileukemic activities and inhibits FLT3 and miR-155 expressions in acute myeloid leukemia.J Hematol Oncol. 2013 Mar 16;6:21. doi: 10.1186/1756-8722-6-21. J Hematol Oncol. 2013. PMID: 23497456 Free PMC article.
-
Inhibition of NEDD8-activating enzyme: a novel approach for the treatment of acute myeloid leukemia.Blood. 2010 May 6;115(18):3796-800. doi: 10.1182/blood-2009-11-254862. Epub 2010 Mar 4. Blood. 2010. PMID: 20203261
-
MLN4924, a NEDD8-activating enzyme inhibitor, is active in diffuse large B-cell lymphoma models: rationale for treatment of NF-{kappa}B-dependent lymphoma.Blood. 2010 Sep 2;116(9):1515-23. doi: 10.1182/blood-2010-03-272567. Epub 2010 Jun 4. Blood. 2010. PMID: 20525923
-
Regulation of the MIR155 host gene in physiological and pathological processes.Gene. 2013 Dec 10;532(1):1-12. doi: 10.1016/j.gene.2012.12.009. Epub 2012 Dec 14. Gene. 2013. PMID: 23246696 Review.
-
Neddylation-Independent Activities of MLN4924.Adv Exp Med Biol. 2020;1217:363-372. doi: 10.1007/978-981-15-1025-0_21. Adv Exp Med Biol. 2020. PMID: 31898238 Review.
Cited by
-
Targeting cullin neddylation for cancer and fibrotic diseases.Theranostics. 2023 Sep 4;13(14):5017-5056. doi: 10.7150/thno.78876. eCollection 2023. Theranostics. 2023. PMID: 37771770 Free PMC article. Review.
-
Strategy for Leukemia Treatment Targeting SHP-1,2 and SHIP.Front Cell Dev Biol. 2021 Aug 19;9:730400. doi: 10.3389/fcell.2021.730400. eCollection 2021. Front Cell Dev Biol. 2021. PMID: 34490276 Free PMC article. Review.
-
Functionally distinct roles for different miR-155 expression levels through contrasting effects on gene expression, in acute myeloid leukaemia.Leukemia. 2017 Apr;31(4):808-820. doi: 10.1038/leu.2016.279. Epub 2016 Oct 14. Leukemia. 2017. PMID: 27740637
-
miR-155 promotes FLT3-ITD-induced myeloproliferative disease through inhibition of the interferon response.Blood. 2017 Jun 8;129(23):3074-3086. doi: 10.1182/blood-2016-09-740209. Epub 2017 Apr 21. Blood. 2017. PMID: 28432220 Free PMC article.
-
Therapeutic Effects of a NEDD8-Activating Enzyme Inhibitor, Pevonedistat, on Sclerodermatous Graft-versus-Host Disease in Mice.Biol Blood Marrow Transplant. 2017 Jan;23(1):30-37. doi: 10.1016/j.bbmt.2016.10.022. Epub 2016 Nov 1. Biol Blood Marrow Transplant. 2017. PMID: 27815049 Free PMC article.
References
-
- Burnett A, Wetzler M, Löwenberg B. Therapeutic advances in acute myeloid leukemia. J Clin Oncol. 2011;29:487–494. - PubMed
-
- Faraoni I, Antonetti FR, Cardone J, Bonmassar E. miR-155 gene: a typical multi-functional microRNA. Biochim Biophys Acta. 2009;1792:497–505. - PubMed
-
- Whitman SP, Maharry K, Radmacher MD, Becker H, Mrózek K, Margeson D, et al. FLT3 internal tandem duplication associates with adverse outcome and gene- and microRNA-expression signatures in patients 60 years of age or older with primary cytogenetically normal acute myeloid leukemia: a Cancer and Leukemia Group B study. Blood. 2010;116:3622–3626. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

