Partial loss of the DNA repair scaffolding protein, Xrcc1, results in increased brain damage and reduced recovery from ischemic stroke in mice
- PMID: 25971543
- PMCID: PMC5576895
- DOI: 10.1016/j.neurobiolaging.2015.04.004
Partial loss of the DNA repair scaffolding protein, Xrcc1, results in increased brain damage and reduced recovery from ischemic stroke in mice
Abstract
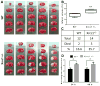
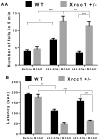
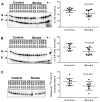
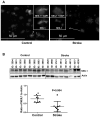
Oxidative DNA damage is mainly repaired by base excision repair (BER). Previously, our laboratory showed that mice lacking the BER glycosylases 8-oxoguanine glycosylase 1 (Ogg1) or nei endonuclease VIII-like 1 (Neil1) recover more poorly from focal ischemic stroke than wild-type mice. Here, a mouse model was used to investigate whether loss of 1 of the 2 alleles of X-ray repair cross-complementing protein 1 (Xrcc1), which encodes a nonenzymatic scaffold protein required for BER, alters recovery from stroke. Ischemia and reperfusion caused higher brain damage and lower functional recovery in Xrcc1(+/-) mice than in wild-type mice. Additionally, a greater percentage of Xrcc1(+/-) mice died as a result of the stroke. Brain samples from human individuals who died of stroke and individuals who died of non-neurological causes were assayed for various steps of BER. Significant losses of thymine glycol incision, abasic endonuclease incision, and single nucleotide incorporation activities were identified, as well as lower expression of XRCC1 and NEIL1 proteins in stroke brains compared with controls. Together, these results suggest that impaired BER is a risk factor in ischemic brain injury and contributes to its recovery.
Keywords: Base excision repair; Oxidative stress; Stroke; Xrcc1.
Published by Elsevier Inc.
Conflict of interest statement
Authors have no conflicts of interest
Figures



Similar articles
-
Single nucleotide polymorphisms of DNA base-excision repair genes (APE1, OGG1 and XRCC1) associated with breast cancer risk in a Chinese population.Asian Pac J Cancer Prev. 2014;15(3):1133-40. doi: 10.7314/apjcp.2014.15.3.1133. Asian Pac J Cancer Prev. 2014. PMID: 24606430
-
Association of DNA base-excision repair XRCC1, OGG1 and APE1 gene polymorphisms with nasopharyngeal carcinoma susceptibility in a Chinese population.Asian Pac J Cancer Prev. 2013;14(9):5145-51. doi: 10.7314/apjcp.2013.14.9.5145. Asian Pac J Cancer Prev. 2013. PMID: 24175791
-
Alzheimer's disease-associated polymorphisms in human OGG1 alter catalytic activity and sensitize cells to DNA damage.Free Radic Biol Med. 2013 Oct;63:115-25. doi: 10.1016/j.freeradbiomed.2013.05.010. Epub 2013 May 14. Free Radic Biol Med. 2013. PMID: 23684897 Free PMC article.
-
New paradigms in the repair of oxidative damage in human genome: mechanisms ensuring repair of mutagenic base lesions during replication and involvement of accessory proteins.Cell Mol Life Sci. 2015 May;72(9):1679-98. doi: 10.1007/s00018-014-1820-z. Epub 2015 Jan 10. Cell Mol Life Sci. 2015. PMID: 25575562 Free PMC article. Review.
-
X-ray repair cross complementing protein 1 in base excision repair.Int J Mol Sci. 2012 Dec 17;13(12):17210-29. doi: 10.3390/ijms131217210. Int J Mol Sci. 2012. PMID: 23247283 Free PMC article. Review.
Cited by
-
Sustained Energy Deficit Following Perinatal Asphyxia: A Shift towards the Fructose-2,6-bisphosphatase (TIGAR)-Dependent Pentose Phosphate Pathway and Postnatal Development.Antioxidants (Basel). 2021 Dec 29;11(1):74. doi: 10.3390/antiox11010074. Antioxidants (Basel). 2021. PMID: 35052577 Free PMC article. Review.
-
The base excision repair process: comparison between higher and lower eukaryotes.Cell Mol Life Sci. 2021 Dec;78(24):7943-7965. doi: 10.1007/s00018-021-03990-9. Epub 2021 Nov 3. Cell Mol Life Sci. 2021. PMID: 34734296 Free PMC article. Review.
-
The Chemical Basis of Intracerebral Hemorrhage and Cell Toxicity With Contributions From Eryptosis and Ferroptosis.Front Cell Neurosci. 2020 Dec 8;14:603043. doi: 10.3389/fncel.2020.603043. eCollection 2020. Front Cell Neurosci. 2020. PMID: 33363457 Free PMC article.
-
Integrative biochemical, proteomics and metabolomics cerebrospinal fluid biomarkers predict clinical conversion to multiple sclerosis.Brain Commun. 2021 Apr 19;3(2):fcab084. doi: 10.1093/braincomms/fcab084. eCollection 2021. Brain Commun. 2021. PMID: 33997784 Free PMC article.
-
The Long-Term Impairment in Redox Homeostasis Observed in the Hippocampus of Rats Subjected to Global Perinatal Asphyxia (PA) Implies Changes in Glutathione-Dependent Antioxidant Enzymes and TIGAR-Dependent Shift Towards the Pentose Phosphate Pathways: Effect of Nicotinamide.Neurotox Res. 2019 Oct;36(3):472-490. doi: 10.1007/s12640-019-00064-4. Epub 2019 Jun 11. Neurotox Res. 2019. PMID: 31187430
References
-
- Bederson JB, Pitts LH, Tsuji M, Nishimura MC, Davis RL, Bartkowski H. Rat middle cerebral artery occlusion: evaluation of the model and development of a neurologic examination. Stroke; a journal of cerebral circulation. 1986;17(3):472–6. - PubMed
-
- Borlongan CV, Yamamoto M, Takei N, Kumazaki M, Ungsuparkorn C, Hida H, Sanberg PR, Nishino H. Glial cell survival is enhanced during melatonin-induced neuroprotection against cerebral ischemia. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2000;14(10):1307–17. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

