Adolescent Alcohol Exposure Amplifies the Incentive Value of Reward-Predictive Cues Through Potentiation of Phasic Dopamine Signaling
- PMID: 25971592
- PMCID: PMC4864623
- DOI: 10.1038/npp.2015.139
Adolescent Alcohol Exposure Amplifies the Incentive Value of Reward-Predictive Cues Through Potentiation of Phasic Dopamine Signaling
Abstract
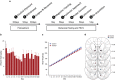
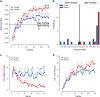
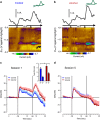
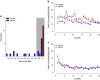
Adolescent alcohol use remains a major public health concern due in part to well-established findings implicating the age of onset in alcohol use in the development of alcohol use disorders and persistent decision-making deficits in adults. We have previously demonstrated that moderate adolescent alcohol consumption in rats promotes suboptimal decision making and an associated perturbation in mesolimbic dopamine transmission in adulthood. Dopamine-dependent incentive learning processes are an integral component of value-based decision making and a fundamental element to many theoretical accounts of addiction. Thus we tested the hypothesis that adolescent alcohol use selectively alters incentive learning processes through perturbation of mesolimbic dopamine systems. To assess incentive learning, behavioral and neurochemical measurements were made during the acquisition, maintenance, extinction, and reacquisition of a Pavlovian conditioned approach procedure in adult rats with a history of adolescent alcohol consumption. We show that moderate adolescent alcohol consumption potentiates stimulus-evoked phasic dopamine transmission, measured in vivo by fast-scan cyclic voltammetry, in adulthood and biases individuals toward a dopamine-dependent incentive learning strategy. Moreover, we demonstrate that animals exposed to alcohol in adolescence are more sensitive to an unexpected variation in reward outcomes. This pattern of phasic dopamine signaling and the associated bias in learning may provide a mechanism for the well-documented vulnerability of individuals with early-life alcohol use for alcohol use disorders in adulthood.
Figures


Similar articles
-
Differential Dopamine Release Dynamics in the Nucleus Accumbens Core and Shell Reveal Complementary Signals for Error Prediction and Incentive Motivation.J Neurosci. 2015 Aug 19;35(33):11572-82. doi: 10.1523/JNEUROSCI.2344-15.2015. J Neurosci. 2015. PMID: 26290234 Free PMC article.
-
Ethanol Exposure History and Alcoholic Reward Differentially Alter Dopamine Release in the Nucleus Accumbens to a Reward-Predictive Cue.Alcohol Clin Exp Res. 2018 Jun;42(6):1051-1061. doi: 10.1111/acer.13636. Epub 2018 Apr 30. Alcohol Clin Exp Res. 2018. PMID: 29602178 Free PMC article.
-
Repeated alcohol administration during adolescence causes changes in the mesolimbic dopaminergic and glutamatergic systems and promotes alcohol intake in the adult rat.J Neurochem. 2009 Feb;108(4):920-31. doi: 10.1111/j.1471-4159.2008.05835.x. Epub 2008 Dec 10. J Neurochem. 2009. PMID: 19077056
-
Nucleus accumbens shell and core dopamine: differential role in behavior and addiction.Behav Brain Res. 2002 Dec 2;137(1-2):75-114. doi: 10.1016/s0166-4328(02)00286-3. Behav Brain Res. 2002. PMID: 12445717 Review.
-
Alcohol during adolescence selectively alters immediate and long-term behavior and neurochemistry.Alcohol. 2010 Feb;44(1):57-66. doi: 10.1016/j.alcohol.2009.09.035. Alcohol. 2010. PMID: 20113874 Free PMC article. Review.
Cited by
-
Voluntary alcohol consumption during distinct phases of adolescence differentially alters adult fear acquisition, extinction and renewal in male and female rats.bioRxiv [Preprint]. 2023 Oct 5:2023.10.03.560757. doi: 10.1101/2023.10.03.560757. bioRxiv. 2023. Update in: Stress. 2023 Nov;26(1):2278315. doi: 10.1080/10253890.2023.2278315. PMID: 37873067 Free PMC article. Updated. Preprint.
-
Reversal of Alcohol-Induced Dysregulation in Dopamine Network Dynamics May Rescue Maladaptive Decision-making.J Neurosci. 2016 Mar 30;36(13):3698-708. doi: 10.1523/JNEUROSCI.4394-15.2016. J Neurosci. 2016. PMID: 27030756 Free PMC article.
-
Sexually dimorphic development of the mesolimbic dopamine system is associated with nuanced sensitivity to adolescent alcohol use.Front Behav Neurosci. 2023 Feb 22;17:1124979. doi: 10.3389/fnbeh.2023.1124979. eCollection 2023. Front Behav Neurosci. 2023. PMID: 36910128 Free PMC article.
-
Mechanisms of Persistent Neurobiological Changes Following Adolescent Alcohol Exposure: NADIA Consortium Findings.Alcohol Clin Exp Res. 2019 Sep;43(9):1806-1822. doi: 10.1111/acer.14154. Epub 2019 Aug 14. Alcohol Clin Exp Res. 2019. PMID: 31335972 Free PMC article. Review.
-
Recruitment and disruption of ventral pallidal cue encoding during alcohol seeking.Eur J Neurosci. 2019 Nov;50(9):3428-3444. doi: 10.1111/ejn.14527. Epub 2019 Aug 19. Eur J Neurosci. 2019. PMID: 31338915 Free PMC article.
References
-
- Alaux-Cantin S, Warnault V, Legastelois R, Botia B, Pierrefiche O, Vilpoux C et al (2013). Alcohol intoxications during adolescence increase motivation for alcohol in adult rats and induce neuroadaptations in the nucleus accumbens. Neuropharmacology 67: 521–531. - PubMed
-
- Baker TE, Stockwell T, Barnes G, Holroyd CB (2011). Individual differences in substance dependence: at the intersection of brain, behaviour and cognition. Addict Biol 16: 458–466. - PubMed
-
- Bickel WK, Marsch LA (2001). Toward a behavioral economic understanding of drug dependence: delay discounting processes. Addiction 96: 73–86. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

