The cerebral embolism evoked by intra-arterial delivery of allogeneic bone marrow mesenchymal stem cells in rats is related to cell dose and infusion velocity
- PMID: 25971703
- PMCID: PMC4429328
- DOI: 10.1186/scrt544
The cerebral embolism evoked by intra-arterial delivery of allogeneic bone marrow mesenchymal stem cells in rats is related to cell dose and infusion velocity
Abstract
Introduction: Intra-arterial cell infusion is an efficient delivery route with which to target organs such as the ischemic brain. However, adverse events including microembolisms and decreased cerebral blood flow were recently reported after intra-arterial cell delivery in rodent models, raising safety concerns. We tested the hypothesis that cell dose, infusion volume, and velocity would be related to the severity of complications after intra-arterial cell delivery.
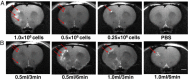
Methods: In this study, 38 rats were subjected to a sham middle cerebral artery occlusion (sham-MCAO) procedure before being infused with allogeneic bone-marrow mesenchymal stem cells at different cell doses (0 to 1.0 × 10(6)), infusion volumes (0.5 to 1.0 ml), and infusion times (3 to 6 minutes). An additional group (n = 4) was infused with 1.0 × 10(6) cells labeled with iron oxide for in vivo tracking of cells. Cells were infused through the external carotid artery under laser Doppler flowmetry monitoring 48 hours after sham-MCAO. Magnetic resonance imaging (MRI) was performed 24 hours after cell infusion to reveal cerebral embolisms or hemorrhage. Limb placing, cylinder, and open field tests were conducted to assess sensorimotor functions before the rats were perfused for histology.
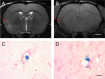
Results: A cell dose-related reduction in cerebral blood flow was noted, as well as an increase in embolic events and concomitant lesion size, and sensorimotor impairment. In addition, a low infusion velocity (0.5 ml/6 minutes) was associated with high rate of complications. Lesions on MRI were confirmed with histology and corresponded to necrotic cell loss and blood-brain barrier leakage.
Conclusions: Particularly cell dose but also infusion velocity contribute to complications encountered after intra-arterial cell transplantation. This should be considered before planning efficacy studies in rats and, potentially, in patients with stroke.
Figures




References
-
- Del Zoppo GJ, Saver JL, Jauch EC, Adams HP, Jr, American Heart Association Stroke Council Expansion of the time window for treatment of acute ischemic stroke with intravenous tissue plasminogen activator: a science advisory from the American Heart Association/American Stroke Association. Stroke. 2009;40:2945–8. doi: 10.1161/STROKEAHA.109.192535. - DOI - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

