Mechanotransduction Mechanisms for Intraventricular Diastolic Vortex Forces and Myocardial Deformations: Part 2
- PMID: 25971844
- PMCID: PMC4519381
- DOI: 10.1007/s12265-015-9630-8
Mechanotransduction Mechanisms for Intraventricular Diastolic Vortex Forces and Myocardial Deformations: Part 2
Abstract
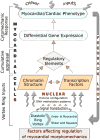
Epigenetic mechanisms are fundamental in cardiac adaptations, remodeling, reverse remodeling, and disease. A primary goal of translational cardiovascular research is recognizing whether disease-related changes in phenotype can be averted by eliminating or reducing the effects of environmental epigenetic risks. There may be significant medical benefits in using gene-by-environment interaction knowledge to prevent or reverse organ abnormalities and disease. This survey proposes that "environmental" forces associated with diastolic RV/LV rotatory flows exert important, albeit still unappreciated, epigenetic actions influencing functional and morphological cardiac adaptations. Mechanisms analogous to Murray's law of hydrodynamic shear-induced endothelial cell modulation of vascular geometry are likely to link diastolic vortex-associated shear, torque and "squeeze" forces to RV/LV adaptations. The time has come to explore a new paradigm in which such forces play a fundamental epigenetic role, and to work out how heart cells react to them. Findings from various imaging modalities, computational fluid dynamics, molecular cell biology and cytomechanics are considered. The following are examined, among others: structural dynamics of myocardial cells (endocardium, cardiomyocytes, and fibroblasts), cytoskeleton, nucleoskeleton, and extracellular matrix; mechanotransduction and signaling; and mechanical epigenetic influences on genetic expression. To help integrate and focus relevant pluridisciplinary research, rotatory RV/LV filling flow is placed within a working context that has a cytomechanics perspective. This new frontier in cardiac research should uncover versatile mechanistic insights linking filling vortex patterns and attendant forces to variable expressions of gene regulation in RV/LV myocardium. In due course, it should reveal intrinsic homeostatic arrangements that support ventricular myocardial function and adaptability.
Figures




References
-
- Pasipoularides A. Heart's Vortex: Intracardiac Blood Flow Phenomena. Shelton, CT: People's Medical Publishing House; 2010. p. 960.
-
- Hines LM, Stampfer MJ, Ma J, et al. Genetic variation in alcohol dehydrogenase and the beneficial effect of moderate alcohol consumption on myocardial infarction. N Engl J Med. 2001;344:549–555. - PubMed
-
- Talmud PJ, Stephens JW, Hawe E, et al. The significant increase in cardiovascular disease risk in APOEepsilon4 carriers is evident only in men who smoke: potential relationship between reduced antioxidant status and ApoE4. Ann Hum Genet. 2005;69:613–622. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

