Development of monosodium acetate-induced osteoarthritis and inflammatory pain in ageing mice
- PMID: 25971876
- PMCID: PMC4430498
- DOI: 10.1007/s11357-015-9792-y
Development of monosodium acetate-induced osteoarthritis and inflammatory pain in ageing mice
Abstract
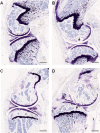
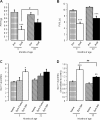
Most conditions associated with ageing result from an age-related loss in the function of cells and tissues that maintain body homeostasis. In osteoarthritis (OA) patients, an inadequate response to stress or joint injury can lead to tissue destruction which can result in chronic pain. Here, we evaluated the development of monoiodoacetate (MIA)-induced OA in 3-, 15- and 22-month-old mice and assessed the pain-like behaviours and the spinal microglial changes associated with MIA administration. We observed that in aged mice, nocifensive behaviour was significantly attenuated in comparison to young adults despite similar knee joint pathology. Specifically referred mechanical allodynia associated with the MIA initial inflammatory phase (0-10 days) was significantly attenuated in 22-month-old mice. In contrast, the late phase of MIA-induced mechanical allodynia was comparable between age groups. Significant increase of microglia cell numbers was detected in 3, but not 15- and 22-month-old spinal cords. Furthermore, in the zymosan model of acute inflammation, mechanical allodynia was attenuated, and microglial response was less robust in 22 compared to 3-month-old mice. This study suggests that nocifensive responses to damaging stimuli are altered with advancing age and microglial response to peripheral damage is less robust.
Figures



References
-
- Bove SE, Calcaterra SL, Brooker RM, Huber CM, Guzman RE, Juneau PL, Schrier DJ, Kilgore KS. Weight bearing as a measure of disease progression and efficacy of anti-inflammatory compounds in a model of monosodium iodoacetate-induced osteoarthritis. Osteoarthr Cartil. 2003;11:821–830. doi: 10.1016/S1063-4584(03)00163-8. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
