Estrogen Regulates Angiotensin II Receptor Expression Patterns and Protects the Heart from Ischemic Injury in Female Rats
- PMID: 25972014
- PMCID: PMC4706310
- DOI: 10.1095/biolreprod.115.129619
Estrogen Regulates Angiotensin II Receptor Expression Patterns and Protects the Heart from Ischemic Injury in Female Rats
Abstract
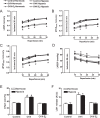
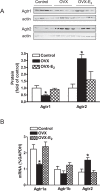
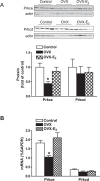
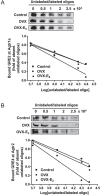
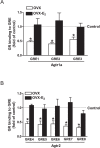
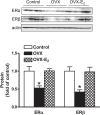
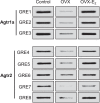
Previous studies have shown that female offspring are resistant to fetal stress-induced programming of ischemic-sensitive phenotype in the heart; however, the mechanisms responsible remain unclear. The present study tested the hypothesis that estrogen plays a role in protecting females in fetal programming of increased heart vulnerability. Pregnant rats were divided into normoxic and hypoxic (10.5% O2 from Day 15 to 21 of gestation) groups. Ovariectomy (OVX) and estrogen (E2) replacement were performed in 8-wk-old female offspring. Hearts of 4-mo-old females were subjected to ischemia and reperfusion injury in a Langendorff preparation. OVX significantly decreased postischemic recovery of left ventricular function and increased myocardial infarction, and no difference was observed between normoxic and hypoxic groups. The effect of OVX was rescued by E2 replacement. OVX decreased the binding of glucocorticoid receptor (GR) to glucocorticoid response elements at angiotensin II type 1 (Agtr1) and type 2 (Agtr2) receptor promoters, resulting in a decrease in Agtr1 and an increase in Agtr2 in the heart. Additionally, OVX decreased estrogen receptor (ER) expression in the heart and inhibited ER/GR interaction in binding to glucocorticoid response elements at the promoters. Consistent with the changes in Agtrs, OVX significantly decreased Prkce abundance in the heart. These OVX-induced changes were abrogated by E2 replacement. The results indicate that estrogen is not directly responsible for the sex dimorphism in fetal programming of heart ischemic vulnerability but suggest a novel mechanism of estrogen in regulating cardiac Agtr1/Agtr2 expression patterns and protecting female hearts against ischemia and reperfusion injury.
Keywords: angiotensin II receptor; estrogen; heart; ischemic injury.
© 2015 by the Society for the Study of Reproduction, Inc.
Figures
Similar articles
-
Maternal High-Fat Diet Causes a Sex-Dependent Increase in AGTR2 Expression and Cardiac Dysfunction in Adult Male Rat Offspring.Biol Reprod. 2015 Aug;93(2):49. doi: 10.1095/biolreprod.115.129916. Epub 2015 Jul 8. Biol Reprod. 2015. PMID: 26157067
-
Glucocorticoid modulates angiotensin II receptor expression patterns and protects the heart from ischemia and reperfusion injury.PLoS One. 2014 Sep 29;9(9):e106827. doi: 10.1371/journal.pone.0106827. eCollection 2014. PLoS One. 2014. PMID: 25265380 Free PMC article.
-
Estrogen normalizes maternal HFD-induced cardiac hypertrophy in offspring by regulating AT2R.J Endocrinol. 2021 Jun 16;250(1):1-12. doi: 10.1530/JOE-20-0562. J Endocrinol. 2021. PMID: 33970125
-
Estradiol attenuates angiotensin-induced aldosterone secretion in ovariectomized rats.Endocrinology. 2000 Dec;141(12):4629-36. doi: 10.1210/endo.141.12.7822. Endocrinology. 2000. PMID: 11108277
-
Non-genomic Effects of Estrogen on Cell Homeostasis and Remodeling With Special Focus on Cardiac Ischemia/Reperfusion Injury.Front Endocrinol (Lausanne). 2019 Oct 25;10:733. doi: 10.3389/fendo.2019.00733. eCollection 2019. Front Endocrinol (Lausanne). 2019. PMID: 31708877 Free PMC article. Review.
Cited by
-
The counter regulatory axis of the renin angiotensin system in the brain and ischaemic stroke: Insight from preclinical stroke studies and therapeutic potential.Cell Signal. 2020 Dec;76:109809. doi: 10.1016/j.cellsig.2020.109809. Epub 2020 Oct 13. Cell Signal. 2020. PMID: 33059037 Free PMC article. Review.
-
Estrogen Contributions to Microvascular Dysfunction Evolving to Heart Failure With Preserved Ejection Fraction.Front Endocrinol (Lausanne). 2019 Jul 3;10:442. doi: 10.3389/fendo.2019.00442. eCollection 2019. Front Endocrinol (Lausanne). 2019. PMID: 31333587 Free PMC article. Review.
-
Prenatal Hypoxia Affects Foetal Cardiovascular Regulatory Mechanisms in a Sex- and Circadian-Dependent Manner: A Review.Int J Mol Sci. 2022 Mar 7;23(5):2885. doi: 10.3390/ijms23052885. Int J Mol Sci. 2022. PMID: 35270026 Free PMC article. Review.
-
Gestational Hypoxia and Developmental Plasticity.Physiol Rev. 2018 Jul 1;98(3):1241-1334. doi: 10.1152/physrev.00043.2017. Physiol Rev. 2018. PMID: 29717932 Free PMC article. Review.
-
The molecular mechanisms in prenatal drug exposure-induced fetal programmed adult cardiovascular disease.Front Pharmacol. 2023 Apr 20;14:1164487. doi: 10.3389/fphar.2023.1164487. eCollection 2023. Front Pharmacol. 2023. PMID: 37153765 Free PMC article. Review.
References
-
- Barker DJ, Osmond C. Infant mortality, childhood nutrition, and ischaemic heart disease in England and Wales. Lancet. 1986;1:1077–1081. - PubMed
-
- Bateson P, Barker D, Clutton-Brock T, Deb D, D'Udine B, Foley RA, Gluckman P, Godfrey K, Kirkwood T, Lahr MM, McNamara J, Metcalfe NB, et al. Developmental plasticity and human health. Nature. 2004;430:419–421. - PubMed
-
- McMillen IC, Robinson JS. Developmental origins of the metabolic syndrome: prediction, plasticity, and programming. Physiol Rev. 2005;85:571–633. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

