Neural mechanisms of placebo anxiolysis
- PMID: 25972166
- PMCID: PMC6705432
- DOI: 10.1523/JNEUROSCI.4793-14.2015
Neural mechanisms of placebo anxiolysis
Abstract
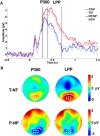
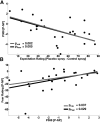
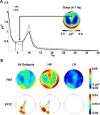
The beneficial effects of placebo treatments on fear and anxiety (placebo anxiolysis) are well known from clinical practice, and there is strong evidence indicating a contribution of treatment expectations to the efficacy of anxiolytic drugs. Although clinically highly relevant, the neural mechanisms underlying placebo anxiolysis are poorly understood. In two studies in humans, we tested whether the administration of an inactive treatment along with verbal suggestions of anxiolysis can attenuate experimentally induced states of phasic fear and/or sustained anxiety. Phasic fear is the response to a well defined threat and includes attentional focusing on the source of threat and concomitant phasic increases of autonomic arousal, whereas in sustained states of anxiety potential and unclear danger requires vigilant scanning of the environment and elevated tonic arousal levels. Our placebo manipulation consistently reduced vigilance measured in terms of undifferentiated reactivity to salient cues (indexed by subjective ratings, skin conductance responses and EEG event-related potentials) and tonic arousal [indexed by cue-unrelated skin conductance levels and enhanced EEG alpha (8-12 Hz) activity], indicating a downregulation of sustained anxiety rather than phasic fear. We also observed a placebo-dependent sustained increase of frontal midline EEG theta (4-7 Hz) power and frontoposterior theta coupling, suggesting the recruitment of frontally based cognitive control functions. Our results thus support the crucial role of treatment expectations in placebo anxiolysis and provide insight into the underlying neural mechanisms.
Keywords: EEG; anxiety; event-related potentials; frontal midline theta; placebo effect.
Copyright © 2015 the authors 0270-6474/15/357365-09$15.00/0.
Figures




References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
