Synapsin determines memory strength after punishment- and relief-learning
- PMID: 25972175
- PMCID: PMC4429153
- DOI: 10.1523/JNEUROSCI.4454-14.2015
Synapsin determines memory strength after punishment- and relief-learning
Abstract
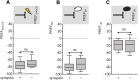
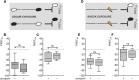
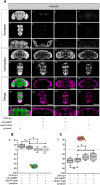
Adverse life events can induce two kinds of memory with opposite valence, dependent on timing: "negative" memories for stimuli preceding them and "positive" memories for stimuli experienced at the moment of "relief." Such punishment memory and relief memory are found in insects, rats, and man. For example, fruit flies (Drosophila melanogaster) avoid an odor after odor-shock training ("forward conditioning" of the odor), whereas after shock-odor training ("backward conditioning" of the odor) they approach it. Do these timing-dependent associative processes share molecular determinants? We focus on the role of Synapsin, a conserved presynaptic phosphoprotein regulating the balance between the reserve pool and the readily releasable pool of synaptic vesicles. We find that a lack of Synapsin leaves task-relevant sensory and motor faculties unaffected. In contrast, both punishment memory and relief memory scores are reduced. These defects reflect a true lessening of associative memory strength, as distortions in nonassociative processing (e.g., susceptibility to handling, adaptation, habituation, sensitization), discrimination ability, and changes in the time course of coincidence detection can be ruled out as alternative explanations. Reductions in punishment- and relief-memory strength are also observed upon an RNAi-mediated knock-down of Synapsin, and are rescued both by acutely restoring Synapsin and by locally restoring it in the mushroom bodies of mutant flies. Thus, both punishment memory and relief memory require the Synapsin protein and in this sense share genetic and molecular determinants. We note that corresponding molecular commonalities between punishment memory and relief memory in humans would constrain pharmacological attempts to selectively interfere with excessive associative punishment memories, e.g., after traumatic experiences.
Keywords: Drosophila; Synapsin; memory; pain; punishment; relief.
Copyright © 2015 Niewalda et al.
Figures







Similar articles
-
Synapsin is required to "boost" memory strength for highly salient events.Learn Mem. 2015 Dec 15;23(1):9-20. doi: 10.1101/lm.039685.115. Print 2016 Jan. Learn Mem. 2015. PMID: 26670182 Free PMC article.
-
Cellular site and molecular mode of synapsin action in associative learning.Learn Mem. 2011 Apr 25;18(5):332-44. doi: 10.1101/lm.2101411. Print 2011. Learn Mem. 2011. PMID: 21518740
-
Independent natural genetic variation of punishment- versus relief-memory.Biol Lett. 2016 Dec;12(12):20160657. doi: 10.1098/rsbl.2016.0657. Biol Lett. 2016. PMID: 28003518 Free PMC article.
-
Maggot learning and Synapsin function.J Exp Biol. 2013 Mar 15;216(Pt 6):939-51. doi: 10.1242/jeb.076208. J Exp Biol. 2013. PMID: 23447663 Review.
-
Pain-relief learning in flies, rats, and man: basic research and applied perspectives.Learn Mem. 2014 Mar 18;21(4):232-52. doi: 10.1101/lm.032995.113. Learn Mem. 2014. PMID: 24643725 Free PMC article. Review.
Cited by
-
Nitric oxide acts as a cotransmitter in a subset of dopaminergic neurons to diversify memory dynamics.Elife. 2019 Nov 14;8:e49257. doi: 10.7554/eLife.49257. Elife. 2019. PMID: 31724947 Free PMC article.
-
Cyclic nucleotide-induced bidirectional long-term synaptic plasticity in Drosophila mushroom body.bioRxiv [Preprint]. 2024 Jan 3:2023.09.28.560058. doi: 10.1101/2023.09.28.560058. bioRxiv. 2024. Update in: J Physiol. 2024 May;602(9):2019-2045. doi: 10.1113/JP285745. PMID: 37808762 Free PMC article. Updated. Preprint.
-
Compromising Tyrosine Hydroxylase Function Extends and Blunts the Temporal Profile of Reinforcement by Dopamine Neurons in Drosophila.J Neurosci. 2025 Mar 12;45(11):e1498242024. doi: 10.1523/JNEUROSCI.1498-24.2024. J Neurosci. 2025. PMID: 39753299
-
Reinforcement signaling of punishment versus relief in fruit flies.Learn Mem. 2018 May 15;25(6):247-257. doi: 10.1101/lm.047308.118. Print 2018 Jun. Learn Mem. 2018. PMID: 29764970 Free PMC article.
-
Reversing Stimulus Timing in Visual Conditioning Leads to Memories with Opposite Valence in Drosophila.PLoS One. 2015 Oct 2;10(10):e0139797. doi: 10.1371/journal.pone.0139797. eCollection 2015. PLoS One. 2015. PMID: 26430885 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
