Neurons forming optic glomeruli compute figure-ground discriminations in Drosophila
- PMID: 25972183
- PMCID: PMC4429157
- DOI: 10.1523/JNEUROSCI.0652-15.2015
Neurons forming optic glomeruli compute figure-ground discriminations in Drosophila
Abstract
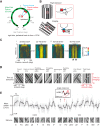
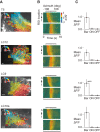
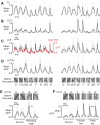
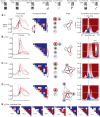
Many animals rely on visual figure-ground discrimination to aid in navigation, and to draw attention to salient features like conspecifics or predators. Even figures that are similar in pattern and luminance to the visual surroundings can be distinguished by the optical disparity generated by their relative motion against the ground, and yet the neural mechanisms underlying these visual discriminations are not well understood. We show in flies that a diverse array of figure-ground stimuli containing a motion-defined edge elicit statistically similar behavioral responses to one another, and statistically distinct behavioral responses from ground motion alone. From studies in larger flies and other insect species, we hypothesized that the circuitry of the lobula--one of the four, primary neuropiles of the fly optic lobe--performs this visual discrimination. Using calcium imaging of input dendrites, we then show that information encoded in cells projecting from the lobula to discrete optic glomeruli in the central brain group these sets of figure-ground stimuli in a homologous manner to the behavior; "figure-like" stimuli are coded similar to one another and "ground-like" stimuli are encoded differently. One cell class responds to the leading edge of a figure and is suppressed by ground motion. Two other classes cluster any figure-like stimuli, including a figure moving opposite the ground, distinctly from ground alone. This evidence demonstrates that lobula outputs provide a diverse basis set encoding visual features necessary for figure detection.
Keywords: feature detection; figure–ground discrimination; optomotor.
Copyright © 2015 the authors 0270-6474/15/357587-13$15.00/0.
Figures



References
-
- Akerboom J, Chen TW, Wardill TJ, Tian L, Marvin JS, Mutlu S, Calderón NC, Esposti F, Borghuis BG, Sun XR, Gordus A, Orger MB, Portugues R, Engert F, Macklin JJ, Filosa A, Aggarwal A, Kerr RA, Takagi R, Kracun S, et al. Optimization of a GCaMP calcium indicator for neural activity imaging. J Neurosci. 2012;32:13819–13840. doi: 10.1523/JNEUROSCI.2601-12.2012. - DOI - PMC - PubMed
-
- Albatineh AN, Niewiadomska-Bugaj M. Correcting Jaccard and other similarity indices for chance agreement in cluster analysis. Adv Data Anal Classif. 2011;5:179–200. doi: 10.1007/s11634-011-0090-y. - DOI
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
