Scatter Photocoagulation Does Not Reduce Macular Edema or Treatment Burden in Patients with Retinal Vein Occlusion: The RELATE Trial
- PMID: 25972260
- PMCID: PMC10020833
- DOI: 10.1016/j.ophtha.2015.04.006
Scatter Photocoagulation Does Not Reduce Macular Edema or Treatment Burden in Patients with Retinal Vein Occlusion: The RELATE Trial
Abstract
Purpose: To determine whether scatter and grid laser photocoagulation (laser) adds benefit to ranibizumab injections in patients with macular edema from retinal vein occlusion (RVO) and to compare 0.5-mg with 2.0-mg ranibizumab.
Design: Randomized, double-masked, controlled clinical trial.
Participants: Thirty-nine patients with central RVO (CRVO) and 42 with branch RVO (BRVO).
Methods: Subjects were randomized to 0.5 mg or 2.0 mg ranibizumab every 4 weeks for 24 weeks and re-randomized to pro re nata ranibizumab plus laser or ranibizumab alone.
Main outcome measures: Mean change from baseline best-corrected visual acuity (BCVA) at week 24 for BCVA at weeks 48, 96, and 144 for second randomization.
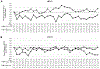
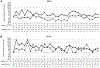
Results: Mean improvement from baseline BCVA at week 24 was 15.5 and 15.8 letters in the 0.5-mg and 2.0-mg CRVO groups, and 12.1 and 14.6 letters in the 0.5-mg and 2.0-mg BRVO groups. For CRVO, but not BRVO, there was significantly greater reduction from baseline mean central subfield thickness (CST) in the 2.0-mg versus 0.5-mg group (396.1 vs. 253.5 μm; P = 0.03). For the second randomization in CRVO patients, there was no significant difference from week 24 BCVA in the ranibizumab plus laser versus the ranibizumab only groups at week 48 (-3.3 vs. 0.0 letters), week 96 (+0.69 vs. -1.6 letters), or week 144 (+0.4 vs. -6.7 letters), and a significant increase from week 24 mean CST at week 48 (+94.7 vs. +15.2 μm; P = 0.05) but not weeks 96 or 144. For BRVO, there was a significant reduction from week 24 mean BCVA in ranibizumab plus laser versus ranibizumab at week 48 (-7.5 vs. +2.8; P < 0.01) and week 96 (-2.0 vs. +4.8; P < 0.03), but not week 144, and there were no differences in mean CST change from week 24 at weeks 48, 96, or 144. Laser failed to increase edema resolution or to reduce the ranibizumab injections between weeks 24 and 144.
Conclusions: In patients with macular edema resulting from RVO, there was no short-term clinically significant benefit from monthly injections of 2.0-mg versus 0.5-mg ranibizumab injections and no long-term benefit in BCVA, resolution of edema, or number of ranibizumab injections obtained by addition of laser treatment to ranibizumab.
Copyright © 2015 American Academy of Ophthalmology. Published by Elsevier Inc. All rights reserved.
Figures

Comment in
-
Reply.Ophthalmology. 2016 May;123(5):e33-4. doi: 10.1016/j.ophtha.2015.11.024. Ophthalmology. 2016. PMID: 27107360 No abstract available.
-
RE: Campochiaro et al.: Scatter photocoagulation does not reduce macular edema or treatment burden in patients with retinal vein occlusion (Ophthalmology 2014;121:209-19).Ophthalmology. 2016 May;123(5):e33. doi: 10.1016/j.ophtha.2015.10.066. Ophthalmology. 2016. PMID: 27107361 No abstract available.
References
-
- Campochiaro PA, Hafiz G, Shah SM, et al. Ranibizumab for macular edema due to retinal vein occlusions; implication of VEGF as a critical stimulator. Molec Ther 2008;16:791–9. - PubMed
-
- Campochiaro PA, Heier JS, Feiner L, et al. Ranibizumab for macular edema following branch retinal vein occlusion: 6-month primary endpoint results of a phase III study. Ophthalmology 2010;117:1102–12. - PubMed
-
- Brown DM, Campochiaro PA, Singh RP, et al. Efficacy and safety of ranibizumab in the treatment of macular edema secondary to central retinal vein occlusion: 6-month results of the phase III CRUISE study. Ophthalmology 2010;117:1124–33. - PubMed
-
- Rosenfeld PJ, Brown DM, Heier JS, et al. Ranibizumab for neovascular age-related macular degeneration. N Engl J Med 2006;355:1419–31. - PubMed
-
- Brown DM, Kaiser PK, Michels M, et al. Ranibizumab versus verteporfin for neovascular age-related macular degeneration. N Engl J Med 2006;355:1432–44. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

